1ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗ
AЈ®ҙЧЛбУл°ұЛ®·ҙУҰЈәCH3COOH+NH3?H2OЁTCH3COO-+NH4+ + H2O
BЈ®ВИЖшёъЛ®·ҙУҰЈәCl2+H2O=2H++Cl-+ClO-
CЈ®ДЖёъЛ®·ҙУҰЈәNa+2H2O=Na++2OH-+H2Ўь
DЈ®БтЛбНӯИЬТәёъЗвСх»ҜұөИЬТә·ҙУҰЈәBa2++SO42-=BaSO4Ўэ
2ЎўСЎФсМв ПВБР»ҜС§·ҪіМКҪЦРЈ¬І»ДЬУГ АлЧУ·ҪіМКҪH++OHЎӘЈҪH2OұнКҫөДКЗ?ЈЁ?Ј©
АлЧУ·ҪіМКҪH++OHЎӘЈҪH2OұнКҫөДКЗ?ЈЁ?Ј©
AЈ®HCI+NaOHЈҪNaCI+H2O
BЈ®Ba(OH)2+2HNO3ЈҪBa(NO3)2+2H2O
CЈ®Cu(OH)2+2HNO3ЈҪCu(NO3)2+2H2O
DЈ®NaOH+NaHSO4=Na2SO4+H2O
3ЎўСЎФсМв ДЬХэИ·ұнКҫПВБР·ҙУҰАлЧУ·ҪіМКҪөДКЗЈЁ?Ј©
AЈ®МъәНПЎСОЛб·ҙУҰЈәFe+2H+=Fe3++H2Ўь
BЈ®МјЛбёЖәНҙЧЛб·ҙУҰЈәCaCO3+2CH3COOH=Ca2++2CH3COO-+H2O+CO2Ўь
CЈ®ЗвСх»ҜұөәНПЎБтЛб·ҙУҰЈәH++OH-=H2O
DЈ®НӯЖ¬УлПЎБтЛб·ҙУҰЈәCu+2H+=Cu2++H2Ўь
4ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪХэИ·өДКЗ
AЈ®NH4C1ИЬТәУлNaOHИЬТә»мәП»мәНЈә OH-+NH4++CI-=NH3ЎӨH2O+Cl-
BЈ®ПЎБтЛбөОФЪНӯЖ¬ЙПЈә Cu+2H+=Cu2++H2Ўь
CЈ®ПЎБтЛбУлЗвСх»ҜұөИЬТә»мәНЈә H++SO42-+Ba2++OH-=BaSO4Ўэ+H2O
DЈ®№эБҝВИЖшНЁИлде»ҜСЗМъИЬТәЦРЈә 3Cl2+4Br-+2Fe2+=2Br2+2Fe3++6CI-
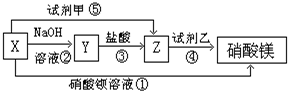
5ЎўМоҝХМв ?XЎўYЎўZИэЦЦОпЦКУРИзПВЧӘ»Ҝ№ШПөЈә
ЈЁ1Ј©ёщҫЭЙПКцЧӘ»Ҝ№ШПөЈ¬РҙіцПВБРОпЦКөД»ҜС§КҪЈәX______ЎўY______ЎўZ______
КФјБјЧ______Ј¬КФјБТТ______
ЈЁ2Ј©РҙіцЙПКцўЫўЬўЭІҪ·ҙУҰөДАлЧУ·ҪіМКҪЈә______Ј®