1ЎўСЎФсМв СОЛбУл№эБҝМъ·ЫөД·ҙУҰЈ¬ИфУРCH3COONa(s,ККБҝ)ҪйИлЈ¬ФтУР
AЈ®·ҙУҰЛЩВКјх»ә
BЈ®ІъЙъЗвЖшөДЧЬБҝІ»ұд
CЈ®ИЬТәөДpHјхРЎ
DЈ®ИЬТәЦРc(OHЈӯ)јхРЎ
ІОҝјҙр°ёЈәAB
ұҫМвҪвОцЈәFeУлСОЛб·ҙУҰөДКөЦКОӘFe+2H+ЁTFe2++H2ЎьЈ¬CH3COONa№ММеУлСОЛб·ҙУҰЙъіЙИхөзҪвЦКCH3COOHЈ¬ИЬТәH+АлЧУЕЁ¶ИҪөөНЈ¬ө«І»ёДұдЗвАлЧУөДОпЦКөДБҝAЈ®ИЬТәH+АлЧУЕЁ¶ИҪөөНЈ¬·ҙУҰЛЩВКјх»әЈ¬№КAХэИ·Ј»BЈ®ЗвАлЧУөДОпЦКөДБҝІ»ёДұдЈ¬ЛщТФІъЙъЗвЖшөДЧЬБҝІ»ұдЈ¬№КBХэИ·Ј»CЈ®ИЬТәH+АлЧУЕЁ¶ИҪөөНЈ¬ИЬТәөДpHФцҙ󣬹КCҙнОуЈ»DЈ®ИЬТәH+АлЧУЕЁ¶ИҪөөНЈ¬ИЬТәЦРcЈЁOH-Ј©Фцҙ󣬹КDҙнОуЈ»№КСЎЈәABЈ®
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ЙиCЈ«CO2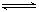 2COЈӯQ1(ОьИИ·ҙУҰ)·ҙУҰЛЩВКОӘ u1Ј¬N2Ј«3H2
2COЈӯQ1(ОьИИ·ҙУҰ)·ҙУҰЛЩВКОӘ u1Ј¬N2Ј«3H2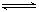 2NH3Ј«Q2(·ЕИИ·ҙУҰ)·ҙУҰЛЩВКОӘ u2Ј¬¶ФУЪЙПКц·ҙУҰЈ¬өұОВ¶ИЙэёЯКұЈ¬u1әНu2өДұд»ҜЗйҝцОӘ?(?)
2NH3Ј«Q2(·ЕИИ·ҙУҰ)·ҙУҰЛЩВКОӘ u2Ј¬¶ФУЪЙПКц·ҙУҰЈ¬өұОВ¶ИЙэёЯКұЈ¬u1әНu2өДұд»ҜЗйҝцОӘ?(?)
AЈ®Н¬ КұФцҙу
BЈ®Н¬ КұјхРЎ
CЈ®ФцҙуЈ¬јхРЎ
DЈ®јхРЎЈ¬Фцҙу
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәЙэёЯОВ¶ИЈ¬»ҜС§·ҙУҰЛЩВКјУҝмЈ¬І»№ЬКЗХэ·ҙУі»№КЗДж·ҙУҰЈ¬№КСЎAЎЈ
өгЖАЈәұҫМвҝјІйөДКЗУ°Пм»ҜС§·ҙУҰЛЩВКөДТтЛШЈ¬МвДҝәЬјтөҘЈ¬Ц»ТӘС§ЙъКмПӨёчёцТтЛШ¶Ф»ҜС§·ҙУҰЛЩВКөДУ°ПмҫНәЬИЭТЧҪвҙрұҫМвЎЈ
ұҫМвДС¶ИЈәјтөҘ
3ЎўСЎФсМв ¶ФУЪA2 + 3B2? ?2CөД·ҙУҰАҙЛөЈ¬ТФПВ»ҜС§·ҙУҰЛЩВКөДұнКҫЦРЈ¬·ҙУҰЛЩВКЧоҝмөДКЗ
?2CөД·ҙУҰАҙЛөЈ¬ТФПВ»ҜС§·ҙУҰЛЩВКөДұнКҫЦРЈ¬·ҙУҰЛЩВКЧоҝмөДКЗ
AЈ®v (B2) =" 0.8mol/" (L?s)? BЈ®v (A2) =" 0.4mol/" (L?s)
CЈ®v (C) =" 0.6mol/" (L?s)? DЈ®v (B2) =" 4.2mol/" (L?min)
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәУЙМвЈ¬Ҫ«СЎПоЦРөД·ҙУҰЛЩВКЧӘ»ҜОӘB2өД·ҙУҰЛЩВКАҙҪшРРұИҪПЈ¬AЈ®v (B2) =" 0.8mol/" (L?s)Ј»|BЈ®v (B2) ="3v" (A2) =" 0.4*3=1.2mol/" (L?s)Ј»CЈ®v (B2)=3/2v (C) =" 0.6*3/2=0.9mol/" (L?s)ЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ТСЦӘДі»ҜС§КөСйөДҪб№ыИзПВұнЈә
КөСйРтәЕ
| ·ҙУҰОп
| ФЪПаН¬ОВ¶ИПВІвөГөД·ҙ
УҰЛЩВКv/molЎӨ(LЎӨmin)-1
|
ҙуРЎПаН¬өДҪрКфЖ¬
| ЛбИЬТә
|
1
| ГҫМх
| 1 molЎӨL-1СОЛб
| v1
|
2
| МъЖ¬
| 1 molЎӨL-1СОЛб
| v2
|
3
| МъЖ¬
| 0.1 molЎӨL-1СОЛб
| v3
|
?ПВБРҪбВЫХэИ·өДКЗ
AЈ®v1>v2>v3? BЈ®v3>v2>v1?CЈ®v1>v3>v2? DЈ®v2>v3>v1
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәҝјІйУ°Пм»ҜС§·ҙУҰЛЩВКөДТтЛШЎЈУ°Пм»ҜС§·ҙУҰЛЩВКөДЦчТӘТтЛШКЗ·ҙУҰОпЧФЙнөДРФЦКЈ¬ГҫұИМъөДҪрКфРФЗҝЈ¬ТтҙЛәНСОЛб·ҙУҰЧоҝмЎЈУЦТтОӘ·ҙУҰОпөДЕЁ¶ИФҪҙуЈ¬·ҙУҰЛЩВКФҪҝмЈ¬ЛщТФХэИ·өДҙр°ёКЗAЎЈ
ұҫМвДС¶ИЈәјтөҘ
5ЎўСЎФсМв Ді·ҙУҰөД·ҙУҰ№эіМЦРДЬБҝұд»ҜИзПВНјЛщКҫ(НјЦРE1ұнКҫХэ·ҙУҰөД»о»ҜДЬЈ¬E2ұнКҫДж·ҙУҰөД»о»ҜДЬ)ЎЈПВБРУР№ШРрКцХэИ·өДКЗ
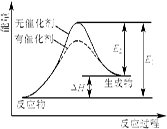
AЈ®ёГ·ҙУҰОӘ·ЕИИ·ҙУҰ
BЈ®ҙЯ»ҜјБДЬёДұд·ҙУҰөДмКұд
CЈ®Дж·ҙУҰөД»о»ҜДЬҙуУЪХэ·ҙУҰөД»о»ҜДЬ
DЈ®ҙЯ»ҜјБДЬҪөөН·ҙУҰөД»о»ҜДЬ