1ЎўјтҙрМв ТСЦӘ·ҙУҰЈә3NO2+H2OЁT2HNO3+NOЈ¬»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ёГ·ҙУҰөДАлЧУ·ҪіМКҪОӘ______Ј®
ЈЁ2Ј©Сх»ҜјБУл»№ФӯјБөДЦКБҝұИОӘ______Ј¬Сх»ҜІъОпУл»№ФӯІъОпөДОпЦКөДБҝЦ®ұИОӘ______Ј®
ЈЁ3Ј©ФЪұкЧјЧҙҝцПВЈ¬3.36L?NO2УлH2OНкИ«·ҙУҰЧӘТЖөДөзЧУКэДҝОӘ______Ј®
ЈЁ4Ј©УГөҘПЯЗЕұкіцёГ·ҙУҰЦРөзЧУЧӘТЖөД·ҪПтәНКэДҝ______Ј®
ЈЁ5Ј©РҙіцHNO3УлКҜ»ТЛ®·ҙУҰөДАлЧУ·ҪіМКҪ______Ј®
ІОҝјҙр°ёЈәЈЁ1Ј©·ҙУҰ3NO2+H2OЁT2HNO3+NOөДАлЧУ·ҪіМКҪОӘ3NO2+H2OЁT2H++2NO3-+NOЈ¬№Кҙр°ёОӘЈә3NO2+H2OЁT2H++2NO3-+NOЈ»
ЈЁ2Ј©·ҙУҰ¶юСх»ҜөӘЦРөӘФӘЛШ»ҜәПјЫјИЙэёЯТІҪөөНЈ¬¶юСх»ҜөӘјИЧчСх»ҜјБУЦЧч»№ФӯјБЈ¬3molөДNO2ЦРЈ¬2molЧц»№ФӯјБЈ¬1molЧцСх»ҜјБЈ¬Сх»ҜјБУл»№ФӯјБөДЦКБҝұИОӘ1Јә2Ј¬
Сх»ҜІъОпКЗ2NO3-Ј¬»№ФӯІъОпКЗNOЈ¬ЛщТФСх»ҜІъОпУл»№ФӯІъОпөДОпЦКөДБҝЦ®ұИОӘ2Јә1Ј¬№Кҙр°ёОӘЈә1Јә2Ј»2Јә1Ј»
ЈЁ3Ј©·ҙУҰ3NO2+H2OЁT2HNO3+NOЧӘТЖөДөзЧУОӘ2molЈ¬јҙ3molөДNO2·ҙУҰЈ¬ЧӘТЖ2molөзЧУЈ¬өұФЪұкЧјЧҙҝцПВЈ¬3.36LЈЁјҙ0.15molЈ©?NO2УлH2OНкИ«·ҙУҰЧӘТЖөДөзЧУОӘ0.1molЈ¬
КэДҝОӘ0.1NAЈ¬№Кҙр°ёОӘЈә0.1NAЈ»
ЈЁ4Ј©ёГ·ҙУҰЦРЈ¬Ц»УРөӘФӯЧУ»ҜәПјЫұд»ҜЈ¬өзЧУЧӘТЖөД·ҪПтәНКэДҝОӘ
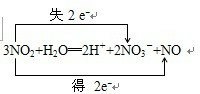
Ј¬№Кҙр°ёОӘЈә

Ј»
ЈЁ5Ј©HNO3ЈЁКЗЗҝЛбЈ©УлКҜ»ТЛ®ЈЁКЗЗҝјоЈ©·ҙУҰЙъіЙПхЛбёЖЈЁҝЙИЬУЪЛ®өДСОЈ©әНЛ®Ј¬АлЧУ·ҪіМКҪОӘH++OH-=H2OЈ¬№Кҙр°ёОӘЈәH++OH-=H2OЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўјтҙрМв РҙіцПВБРАлЧУ·ҪіМКҪ
ЈЁ1Ј©өИМе»эОпЦКөДБҝЕЁ¶ИөДNaHCO3әНBaЈЁOHЈ©2БҪИЬТә»мәП______Ј®
ЈЁ2Ј©БтЛбВБИЬТәУл№эБҝBaЈЁOHЈ©2ИЬТә·ҙУҰ______Ј®
ЈЁ3Ј©ИэВИ»ҜМъЕЁИЬТәөОИл·РЛ®ЦРЈ¬ЦЖИЎЗвСх»ҜМъҪәМе______Ј®
ЈЁ4Ј©НӯЖ¬јУИлЕЁПхЛбЦР______Ј®
ІОҝјҙр°ёЈәЈЁ1Ј©өИМе»эОпЦКөДБҝЕЁ¶ИөДNaHCO3әНBaЈЁOHЈ©2БҪИЬТә»мәПЙъіЙBaCO3әНH2OЈ¬·ҙУҰөДАлЧУ·ҪіМКҪОӘHCO3-+Ba2++OH-=BaCO3Ўэ+H2OЈ¬
№Кҙр°ёОӘЈәHCO3-+Ba2++OH-=BaCO3Ўэ+H2OЈ»
ЈЁ2Ј©БтЛбВБИЬТәУл№эБҝBaЈЁOHЈ©2ИЬТә·ҙУҰЙъіЙBaSO4ЎўAlO2-әНH2OЈ¬·ҙУҰөДАлЧУ·ҪіМКҪОӘ2Al3++3SO42-+3Ba2++8OH-=3BaSO4Ўэ+2AlO2-+4H2OЈ¬
№Кҙр°ёОӘЈә2Al3++3SO42-+3Ba2++8OH-=3BaSO4Ўэ+2AlO2-+4H2OЈ»
ЈЁ3Ј©ИэВИ»ҜМъЕЁИЬТәөОИл·РЛ®ЦРЈ¬МъАлЧУЛ®ҪвЙъіЙЗвСх»ҜМъҪәМеәНH+Ј¬·ҙУҰөДАлЧУ·ҪіМКҪОӘFe3++3H2O?Ўч?.?FeЈЁOHЈ©3ЈЁҪәМеЈ©+3H+Ј¬
№Кҙр°ёОӘЈәFe3++3H2O?Ўч?.?FeЈЁOHЈ©3ЈЁҪәМеЈ©+3H+Ј»
ЈЁ4Ј©НӯЖ¬јУИлЕЁПхЛбЦРЈ¬·ҙУҰЙъіЙCu2+ЎўNO2әНH2OЈ¬·ҙУҰөДАлЧУ·ҪіМКҪОӘCu+2NO3-+4H+=Cu2++2NO2Ўь+2H2OЈ¬
№Кҙр°ёОӘЈәCu+2NO3-+4H+=Cu2++2NO2Ўь+2H2OЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪЦРЈ¬КйРҙХэИ·өДКЗ
[? ]
A. ДЖәНАдЛ®·ҙУҰЈәNa + 2H2O = Na+ + 2OH- +H2Ўь
B. ВБИЬУЪЗвСх»ҜДЖИЬТәЈәAl + 2OH- = AlO2- + H2Ўь
C. ҪрКфВБИЬУЪСОЛбЦРЈә2Al + 6H+ = 2Al3+ + 3H2Ўь
D. МъёъПЎБтЛб·ҙУҰЈә2Fe + 6H+ = 2Fe3+ + 3H2Ўь
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ДЬХэИ·ұнКҫПВБР·ҙУҰөДАлЧУ·ҪіМКҪКЗ?
AЈ®ПтFe(NO3)2ПЎИЬТәЦРјУИлСОЛбЈә3Fe2Ј«Ј«4HЈ«Ј«NO3Јӯ===3Fe3Ј«Ј«NOЎьЈ«2H2O
BЈ®ИэВИ»ҜМъЕЁИЬТәөОИл·РЛ®ЦРЈ¬ЦЖИЎЗвСх»ҜМъҪәМеFe3Ј«Ј«3H2O Fe(OH)3ЎэЈ«3HЈ«
Fe(OH)3ЎэЈ«3HЈ«
CЈ®МјЛбЗвп§ИЬТәУлЧгБҝөДNaOHИЬТә»мәПәујУИИЈәNH4Ј«Ј«OHЈӯ NH3ЎьЈ«H2O
NH3ЎьЈ«H2O
DЈ®ВБЖ¬ёъЗвСх»ҜДЖИЬТә·ҙУҰЈәAlЈ«2OHЈӯЈҪAlO2ЈӯЈ«H2Ўь
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәBПоЦЖИЎҪәМеІ»УҰРҙіБөн·ыәЕЈ¬ҙнОуЈ»CПоТтОӘЧгБҝөДNaOHИЬТәЈ¬ЛщТФУҰОӘNH4Ј«Ј«HCO3-+2OHЈӯ NH3ЎьЈ«2H2O+ CO32-,ҙнОуЈ»DПоУҰОӘ2AlЈ«2H2O+2OHЈӯЈҪ2AlO2ЈӯЈ«3H2ЎьЎЈ
NH3ЎьЈ«2H2O+ CO32-,ҙнОуЈ»DПоУҰОӘ2AlЈ«2H2O+2OHЈӯЈҪ2AlO2ЈӯЈ«3H2ЎьЎЈ
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®іОЗеөДКҜ»ТЛ®УлПЎСОЛб·ҙУҰЈәCaЈЁOHЈ©2+2H+=Ca2++2H2O
BЈ®СЗБтЛбЗвДЖЛ®HSO3-+H2O?SO32-+H3O+
CЈ®Сх»ҜМъИЬУЪЗвөвЛбЈәFe2O3+6H+=2Fe3++3H2O
DЈ®УГКҜД«өзј«өзҪвNaClИЬТәЈә2Cl-+2H2O
2OH-+Cl2Ўь+H2Ўь
ІОҝјҙр°ёЈә
AЈ®АлЧУ·ҪіМКҪЦРУҰҪ«ТЧИЬУЪЛ®Ј¬ТЧөзАлөДЗҝөзҪвЦКІріЙАлЧУРОКҪЈ®ёГСЎПоЦРіОЗеөДКҜ»ТЛ®әНПЎСОЛб¶јКфУЪҙЛАаЈ®№КіОЗеөДКҜ»ТЛ®УлПЎСОЛб·ҙУҰөДАлЧУ·ҪіМКҪОӘЈәH++OH-=H2OЈ®№КAҙнОуЈ»
BЈ®СЗБтЛбКЗ¶юФӘИхЛбЈ¬ёщҫЭСОАаЛ®ҪвөД№жВЙЈ¬СЗБтЛбЗвёщАлЧУЛ®ҪвЈ¬Л®ҪвЙъіЙСЗБтЛбЈ¬ЖдЛ®Ҫв·ҪіМКҪОӘЈәЈәHSO3-+H2O?H2SO3+OH-Ј®№КBҙнОуЈ»
CЈ®Сх»ҜМъУлЗвөвЛбөзАліцөДЗвАлЧУ·ҙУҰЈ¬ЙъіЙ+3јЫөДМъАлЧУЈ¬ө«Fe3+ДЬҪ«ЗвөвЛбөзАліцөД-1јЫөДөвАлЧУСх»ҜЈ¬ЖдХэИ·өДАлЧУ·ҙУҰ·ҪіМКҪОӘЈәFe2O3+6H++2I-=2Fe2++3H2O+I2Ј®№КCҙнОуЈ»
DЈ®УГКҜД«Ччөзј«өзҪвВИ»ҜДЖИЬТәЈ¬Сфј«ОьёҪөДКЗИЬТәЦРөДТхАлЧУCl-ЎўOH-Ј¬Cl-ұИOH-ёьТЧК§ИҘөзЧУЈ¬·ўЙъ·ҙУҰЈә2Cl--2e-=Cl2ЎьЈ¬ЙъіЙВИЖшәНЗвСх»ҜДЖЈ¬Тхј«ОьёҪИЬТәЦРH+әНNa+Ј¬H+УЕПИУЪNa+өГөҪөзЧУЈ¬·ўЙъ·ҙУҰЈә2H++2e-=H2ЎьЈ¬ЙъіЙЗвЖшЈ¬ЛщТФЧЬөДөзј«·ҙУҰОӘЈә2Cl-+2H2O?өзҪв?.?2OH-+Cl2Ўь+H2ЎьЈ¬№КDХэИ·Ј»
№КСЎDЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г