1ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®°СҪрКфМъ·ЕИлПЎБтЛбЦР2Fe+6H+=2Fe3++3H2Ўь
BЈ®МјЛбұөУлСОЛб·ҙУҰЈәBaCO3+2H+=Ba2++CO2Ўь+H2O
CЈ®НщіОЗеКҜ»ТЛ®ЦРөОИлЙЩБҝөДМјЛбЗвДЖИЬТәЈәCa2++OH-+HCO3-ЁTCaCO3Ўэ+H2O
DЈ®ЗвСх»ҜұөИЬТәУлПЎH2SO4·ҙУҰЈәBa2++SO42-=BaSO4Ўэ
ІОҝјҙр°ёЈәAЈ®°СҪрКфМъ·ЕИлПЎБтЛбЦРөДАлЧУ·ҙУҰОӘFe+2H+=Fe2++H2ЎьЈ¬№КAҙнОуЈ»
BЈ®МјЛбұөУлСОЛб·ҙУҰөДАлЧУ·ҙУҰОӘBaCO3+2H+=Ba2++CO2Ўь+H2OЈ¬№КBХэИ·Ј»
CЈ®НщіОЗеКҜ»ТЛ®ЦРөОИлЙЩБҝөДМјЛбЗвДЖИЬТәЈ¬МјЛбЗвДЖНкИ«·ҙУҰЈ¬ФтАлЧУ·ҙУҰОӘCa2++OH-+HCO3-ЁTCaCO3Ўэ+H2OЈ¬№КCХэИ·Ј»
DЈ®ЗвСх»ҜұөИЬТәУлПЎH2SO4·ҙУҰөДАлЧУ·ҙУҰОӘ2OH-+2H++Ba2++SO42-=BaSO4Ўэ+2H2OЈ¬№КDҙнОуЈ»
№КСЎBCЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўМоҝХМв ЈЁ8·ЦЈ©°СПВБРАлЧУ·ҪіМКҪёДРҙіЙ»ҜС§·ҪіМКҪ
ЈЁ1Ј©CO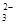 Ј«2HЈ«
Ј«2HЈ«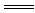 CO2ЎьЈ«H2O??
CO2ЎьЈ«H2O??
ЈЁ2Ј©AgЈ«Ј«ClЈӯ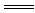 AgClЎэ??
AgClЎэ?? ?
?
ЈЁ3Ј©Cu2Ј«Ј«2OHЈӯ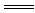 CuЈЁOHЈ©2Ўэ??
CuЈЁOHЈ©2Ўэ??
ЈЁ4Ј©Ba2+К®SO42Јӯ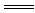 BaSO4
BaSO4 Ўэ??
Ўэ??
ІОҝјҙр°ёЈә
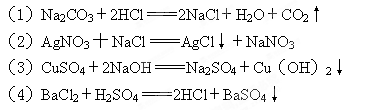
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗ(ЎЎЎЎ)
AЈ®NaClOИЬТәЦРНЁИлЙЩБҝөДSO2ЖшМеЈә2ClOЈӯЈ«SO2Ј«H2O=SO32-Ј«2HClO
BЈ®НӯёъЕЁПхЛб·ҙУҰЈә3CuЈ«8HЈ«Ј«2NO3-=3Cu2Ј«Ј«2NOЎьЈ«4H2O
CЈ®ПтCa(HCO3)2ИЬТәЦРөОјУ№эБҝNaOHИЬТәЈәCa2Ј«Ј«2HCO3-Ј«2OH-=CaCO3ЎэЈ«CO32-Ј«2H2O
DЈ®өИМе»эөИОпЦКөДБҝЕЁ¶ИөДNH4HCO3УлBa(OH)2ИЬТә»мәПәујУИИЈәNH4+Ј«OHЈӯ=NH3ЎьЈ«H2O
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈәҙОВИЛбДЖјИУРСх»ҜРФЈ¬ДЬ°СSO2Сх»ҜЙъіЙБтЛбЈ¬СЎПоAІ»ХэИ·Ј»СЎПоBІ»ХэИ·Ј¬»№ФӯІъОпУҰёГКЗNO2Ј»СЎПоDІ»ХэИ·Ј¬»№УРМјЛбұөіБөнЙъіЙЈ¬ЛщТФХэИ·өДҙр°ёСЎCЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪХэИ·өДКЗЈЁ?Ј©
AЈ®ПтNaHCO3ИЬТәЦРјУИл№эБҝКҜ»ТЛ®Јә2HCO3ЈӯЈ«Ca2Ј«Ј«2OHЈӯ===CaCO3ЎэЈ«CO32ЈӯЈ«2H2O
BЈ®ПтAlCl3ИЬТәЦРөОјУ№эБҝ°ұЛ®ЈәAl3Ј«Ј«4NH3ЎӨH2O="==" AlO2ЈӯЈ«4NH4Ј«Ј«2H2O
CЈ®ВИЖшНЁИлЛ®ЦРЈәCl2Ј«H2O===2HЈ«Ј«ClЈӯЈ«ClOЈӯ
DЈ®FeCl3ИЬТәёҜКҙНӯЦКПЯВ·°еЈәCuЈ«2Fe3Ј«===Cu2Ј«Ј«2Fe2Ј«
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәAПоЈәOHЈӯөД»ҜС§јЖБҝКэУҰОӘ1Ј¬№КҙнЈ»BПоЈәЗвСх»ҜВБІ»ИЬУЪ°ұЛ®Ј¬№КҙнЈ»CПоЈә ·ҙУҰОӘҝЙДж·ҙУҰЈ¬№КҙнЎЈ№КСЎDЎЈ
өгЖАЈәАлЧУ·ҪіМКҪХэОуЕР¶ПТӘЧўТвЈәҝҙФӯФтЈәҝҙКЗ·с·ыәПҝН№ЫКВКөЎўҝҙКЗ·с·ыәПЦКБҝКШәгЎўҝҙКЗ·с·ыәПөзәЙКШәгЎўҝҙКЗ·с·ыәПөГК§өзЧУПаөИЎЈҝЙИЬөДТЧөзАлөДОпЦКІріЙАлЧУЎЈЧўТв№эБҝЎўЙЩБҝЎўККБҝөИЎЈ
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗ
[? ]
AЈ®ЙЩБҝNaHCO3ИЬТәУл№эБҝөДBa(OH)2ИЬТәЈәHCO3-+Ba2++OH-ЈҪBaCO3Ўэ+H2O
BЈ®МјЛбёЖИЬУЪҙЧЛбИЬТәЈәCaCO3+2H+ЈҪCa2++CO2Ўь+H2O
CЈ®Fe3O4ИЬУЪ№эБҝөДПЎHNO3ЈәFe3O4+8H+ЈҪ2Fe3++Fe2++4H2O
DЈ®КөСйКТУГЕЁСОЛбУлMnO2·ҙУҰЦЖCl2ЈәMnO2+2H++2Cl-ЈҪCl2Ўь+Mn2++H2O
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г