1ЎўСЎФсМв НӯРҝФӯөзіШЈЁИзНјЈ©№ӨЧчКұЈ¬ПВБРРрКцҙнОуөДКЗ
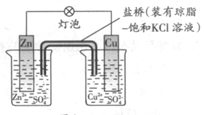
A.Хэј«·ҙУҰОӘЈәCu2++2eЁC= Cu
B.өзіШ·ҙУҰОӘЈәZn+Cu2+=Zn2+ +Cu
C.ФЪНвөзВ·ЦРЈ¬өзЧУҙУёәј«БчПтХэј«
D.СОЗЕЦРөДK+ТЖПтZnSO4ИЬТә
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәКФМв·ЦОцЈәНӯРҝФӯөзіШЦРРҝҪП»оЖГК§ИҘөзЧУ·ўЙъСх»Ҝ·ҙУҰКЗёәј«Ј¬Нӯј«өГөҪөзЧУ·ўЙъ»№Фӯ·ҙУҰКЗХэј«ЎЈУЙУЪРҝј«СфАлЧУЈЁZn2+ Ј©ФҪАҙФҪ¶аЈ¬ОӘБЛО¬іЦИЬТәөзЦРРФЈ¬СОЗЕөДCl-»бТЖПтZnSO4ИЬТәЈ¬¶шІ»КЗK+Ј¬№КСЎD
ҝјөгЈәФӯөзіШ
өгЖАЈәҝјІйФӯөзіШөД»щұҫ№ӨЧчФӯАнЈ¬ДС¶ИІ»ҙуЈ¬КфУЪіЈҝјМвЎЈ
ұҫМвДС¶ИЈәјтөҘ
2ЎўСЎФсМв ПВБР¶БКэІ»ХэИ·өДКЗ( ?)
AЈ®УГ№г·әpHКФЦҪІвөГДіИЬТәөДpHОӘ3.4
BЈ®УГ10 mL БҝНІБҝИЎ8.8 mL NaOHИЬТә
CЈ®УГНРЕММмЖҪіЖБҝ7.9 g NaCl№ММе
DЈ®УГЛбКҪөО¶Ё№ЬБҝИЎ18.00 mL HCl
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈә№г·әpHКФЦҪЦ»ДЬ¶БКэөҪХыКэЈ¬AІ»ХэИ·Ј»БҝНІНРЕММмЖҪҝЙТФ¶БКэөҪ0.1Ј¬BЎўCХэИ·Ј»өО¶Ё№ЬДЬ¶БКэөҪ0.01mlЈ¬СЎПоDХэИ·Ј¬ҙр°ёСЎAЎЈ
өгЖАЈәёГМвКЗ»щҙЎРФКФМвөДҝјІйЈ¬ДС¶ИІ»ҙуЎЈЦчТӘКЗҝјІйС§Йъ¶ФіЈјыТЗЖч¶БКэТФј°¶БКэФӯФтөДБЛҪвХЖОХЗйҝцЈ¬УРАыУЪЕаСшС§ЙъөДЧЫәПКөСйДЬБҰәН№ж·¶СПҪчөДҝЖС§МҪҫҝДЬБҰЎЈ
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв КөСйЦРРиУГ2.0mol/LөДNa2CO3ИЬТә950mLЈ¬ЕдЦЖКұУҰСЎУГөДИЭБҝЖҝөД№жёсәНіЖИЎNa2CO3өДЦКБҝ·ЦұрОӘ(? )
A? 1000mLЈ¬212g?B? 950mLЈ¬201.4g
C? 100mLЈ¬21.2g?D? 500mLЈ¬100.7g
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ
4ЎўКөСйМв ИзПВНјКЗіЈјыТЗЖчөДІҝ·ЦҪб№№ЎЈ
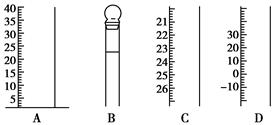
(1)РҙіцТЗЖчГыіЖЈәA________Ј¬B________Ј¬C________Ј¬
(2)К№УГЗ°РијмІйКЗ·сВ©Л®өДТЗЖчУР________ЎЈЈЁМоРтәЕЈ©
ІОҝјҙр°ёЈә(1)AБҝНІЈ¬BИЭБҝЖҝЈ¬CөО¶Ё№ЬЈ¬(2)B? C
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ
5ЎўСЎФсМв ТФПВКЗТ»Р©іЈУГөДОЈПХЖ·ұкЦҫЈ¬ҙж·ЕЗвСх»ҜДЖөДИЭЖчУҰМщөДұкЗ©КЗЈЁ?Ј©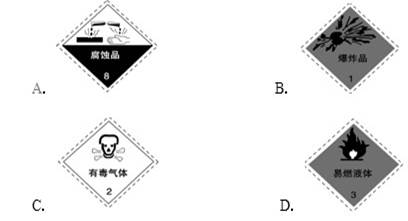
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәҝјІйіЈјыөДОЈПХЖ·ұкЦҫөДК¶ұрУлЕР¶ПЎЈЗвСх»ҜДЖј«ТЧәЬЗҝөДёҜКҙРФЈ¬ЛщТФСЎПоAХэИ·Ј¬ҙр°ёСЎAЎЈ
ұҫМвДС¶ИЈәјтөҘ