1ЎўСЎФсМв Ј®ТСЦӘC6H5ONaЈ«CO2Ј«H2OЁDЎъC6H5OHЈ«NaHCO3Ј¬ДіУР»ъОпөДҪб№№јтКҪИзПВЈә

NaЎўNaOHЎўNaHCO3·ЦұрУлөИОпЦКөДБҝөДёГОпЦКЗЎәГ·ҙУҰКұЈ¬NaЎўNaOHЎўNaHCO 3өДОпЦКөДБҝЦ®ұИОӘ
AЈ®3ЎГ3ЎГ2
BЈ®3ЎГ2ЎГ1
CЈ®1ЎГ1ЎГ1
DЈ®3ЎГ2ЎГ2
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәУлДЖ·ҙУҰөДУРҙјфЗ»щЎў·УфЗ»щЎўфИ»щЈ¬УлЗвСх»ҜДЖ·ҙУҰУР·УфЗ»щЎўфИ»щЈ¬әНМјЛбЗвДЖ·ҙУҰөДЦ»УРфИ»щЈ¬ЛщТФ3ЎГ2ЎГ1
ұҫМвДС¶ИЈәјтөҘ
2ЎўМоҝХМв ЈЁ6·ЦЈ©ФЪ200ml 0.5 molЎӨL-1өДAl2(SO4)3ИЬТәЦРЈ¬Al3+өДОпЦКөДБҝЕЁ¶ИКЗ?Ј¬Al3+өДЦКБҝКЗ?Ј¬SO42-өДёцКэКЗ?ЎЈ
ІОҝјҙр°ёЈә1mol/L?5.4g? 0.6NA
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв Ҫ«ә¬1mol HClөДПЎСОЛбЈ¬ЦрөОјУИлә¬0.2 mol NaOHәН0.5 molNa2CO3өД»мәПИЬТәЦРЈ¬ід·Ц·ҙУҰәуИЬТәЦРёчОпЦКөДБҝКЗ
| HCl/mol
| NaCl/mol
| NaOH/mol
| Na2CO3/mol
| NaHCO3/mol
|
A
| 0
| 1
| 0.1
| 0
| 0.1
|
B
| 0
| 1
| 0
| 0.1
| 0.1
|
C
| 0.2
| 0.8
| 0
| 0
| 0.2
|
D
| 0
| 1
| 0
| 0
| 0.2
|
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәHClКЧПИУлNaOH·ўЙъЦРәН·ҙУҰЈ¬HClФЩәНNa2CO3·ҙУҰЙъіЙNaHCO3Ј¬HClФЩәНNaHCO3·ҙУҰЈ¬ёщҫЭ·ҙУҰҪшРРјЖЛгЎЈ№КСЎDЎЈ
ҝјөгЈәДЖөДЦШТӘ»ҜәПОп »ҜС§јЖЛг
өгЖАЈәұҫМвҝјІйөДКЗОЮ»ъ·ҙУҰөДЦӘК¶әН»ҜС§јЖЛгЈ¬·ЦОцЗеіюHClәНNa2CO3·ҙУҰөДФӯАнКЗҪвМвөД№ШјьЈ¬МвДҝДС¶ИККЦРЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв NAОӘ°ў·ьјУөВВЮіЈКэЈ¬ПВБРЛө·ЁЦРХэИ·өДКЗ(? )
AЈ®17 g 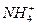 ЦРә¬УРөДөзЧУКэОӘ10NA
ЦРә¬УРөДөзЧУКэОӘ10NA
BЈ®1 molЎӨL-1 Mg(NO3)2ИЬТәЦРә¬УР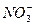 өДКэДҝОӘ2NA
өДКэДҝОӘ2NA
CЈ®ұкЧјЧҙҝцПВЈ¬22.4 LјЧұҪЛщә¬өД·ЦЧУКэОӘNA
DЈ®КТОВПВЈ¬28.0 gТТП©әНұыП©ЦР»мәПЖшМеЦРә¬УРөДМјФӯЧУКэОӘ2NA
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәұҫМвҝјІйОпЦКЦ®јдөД°ьә¬№ШПөЈ¬18 g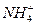 ЦРә¬УРөДөзЧУКэІЕОӘ10NAЈ»BПоГ»УРИЬТәМе»эЈ¬ОЮ·ЁЗуОпЦКөДБҝЈ¬ЛщТФОЮ·ЁЗуКэДҝЈ»CПојЧұҪОӘТәМеЈ¬І»КЗЖшМеЈ¬ЛщТФ22.4 LјЧұҪІўІ»КЗ1 molЈ»ТТП©әНұыП©ЦРФӯЧУёцКэCЎГH=1ЎГ2Ј¬ЛщТФІ»КЬТТП©әНұыП©ұИАэУ°ПмЈ¬Ц»ТӘЦКБҝТ»¶ЁЈ¬28.0 gЦРЈ¬ә¬УРөДМјФӯЧУКэОӘ2NAЎЈ
ЦРә¬УРөДөзЧУКэІЕОӘ10NAЈ»BПоГ»УРИЬТәМе»эЈ¬ОЮ·ЁЗуОпЦКөДБҝЈ¬ЛщТФОЮ·ЁЗуКэДҝЈ»CПојЧұҪОӘТәМеЈ¬І»КЗЖшМеЈ¬ЛщТФ22.4 LјЧұҪІўІ»КЗ1 molЈ»ТТП©әНұыП©ЦРФӯЧУёцКэCЎГH=1ЎГ2Ј¬ЛщТФІ»КЬТТП©әНұыП©ұИАэУ°ПмЈ¬Ц»ТӘЦКБҝТ»¶ЁЈ¬28.0 gЦРЈ¬ә¬УРөДМјФӯЧУКэОӘ2NAЎЈ
ұҫМвДС¶ИЈәјтөҘ
5ЎўСЎФсМв Йи°ў·ьјУөВВЮіЈКэЈЁNAЈ©өДКэЦөОӘnAЈ¬ПВБРЛө·ЁЦРХэИ·өДКЗ
AЈ®1 mol Cl2УлЧгБҝNaOH·ҙУҰЈ¬ЧӘТЖөДөзЧУКэОӘ2nA
BЈ®1 mo1Naұ»НкИ«Сх»ҜЙъіЙNa2O2Ј¬К§ИҘөДөзЧУКэОӘ2nA
CЈ®іЈОВіЈС№ПВЈ¬22Ј®4LSO2ЖшМеЦРә¬УРөДФӯЧУКэРЎУЪ3nA
DЈ®0Ј®1 mol/LNaOHИЬТәЦРә¬УРNa+өДКэДҝОӘ0Ј® 1 nA