1ЎўСЎФсМв ФЪ0.25?mol?Na2OЦРЈ¬ә¬УРөДСхАлЧУКэФјОӘ
A.1ёц
B.0.25ёц
C.1.5ЎБ1023ёц
D.6.02ЎБ1023ёц
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈәСх»ҜДЖЦРСфАлЧУәНТхАлЧУөДёцКэЦ®ұИКЗ2©U1Ј¬ЛщТФә¬УРөДТхАлЧУСхАлЧУКЗ0.25molЈ¬КэДҝКЗ0.25ЎБ6.02ЎБ1023ЈҪ1.5ЎБ1023ёцЈ¬ҙр°ёСЎCЎЈ
ұҫМвДС¶ИЈәјтөҘ
2ЎўСЎФсМв іэИҘПВБРОпЦКЦРә¬УРөДЙЩБҝФУЦКЈЁАЁәЕЦРөДОпЦКЈ© өД·Ҫ·ЁЦРҙнОуөДКЗ?
[? ]
AЈ®H2( HCl)НЁ№эЛ®ПҙөУ
BЈ®NO(NH3)НЁ№эЛ®ПҙөУ
CЈ®NH4Cl( NaCl)јУИИ»мәПОп
DЈ®NH4ClИЬТә(I2)УГCCl4ЭНИЎ
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ҝЙТФУГ·ЦТәВ©¶··ЦАлөДТ»Чй»мәПОпКЗ
AЈ®деәНCCl4
BЈ®ұҪәНдеұҪ
CЈ®деТТНйәНЛ®
DЈ®ҫЖҫ«әНЛ®
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈәЦ»УР»ҘІ»ПаИЬөДТәМеЦ®јдІЕДЬУГ·ЦТәВ©¶·ҪшРР·ЦАлЈ¬деТТНйІ»ИЬУЪЛ®Ј¬CХэИ·ЎЈЖдУа¶јКЗТЧИЬ»т»ҘИЬөДЈ¬І»ХэИ·Ј¬ҙр°ёСЎCЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўКөСйМв (9·Ц)Гҫј°ЖдәПҪрКЗТ»ЦЦУГНҫәЬ№гөДҪрКфІДБПЈ¬әЈЛ®ЦРГҫөДә¬БҝОӘ1.10 mgЎӨLЈӯ1Ј¬ДҝЗ°КАҪзЙП60%өДГҫКЗҙУәЈЛ®ЦРМбИЎөДЎЈЦчТӘІҪЦиИзПВЈә

(1)ОӘБЛК№MgSO4НкИ«ЧӘ»ҜОӘMg(OH)2Ј¬ҝЙТФјУИл№эБҝКФјБўЩЈ¬КФјБўЩЧоәГСЎУГ__________ЎЈ
(2)јУИлКФјБўЩәуЈ¬ДЬ№»·ЦАлөГөҪMg(OH)2іБөнөД·Ҫ·ЁКЗ
________________________________________________________________________ЎЈ
(3)КФјБўЪҝЙТФСЎУГ________Ј¬Жд·ҙУҰөДАлЧУ·ҪіМКҪОӘ_________________________
________________________________________________________________________ЎЈ
(4)ОЮЛ®MgCl2ФЪИЫИЪЧҙМ¬ПВЈ¬НЁөзәу»бІъЙъCl2әНMgЈ¬РҙіцёГ·ҙУҰөД»ҜС§·ҪіМКҪ________________________________________________________________________
________________________________________________________________________ЎЈ
(5)ИфјЩЙиәЈЛ®ЦРГҫФӘЛШИ«ІҝҙжФЪУЪMgSO4ЦРЈ¬ФтәЈЛ®ЦРMgSO4өДә¬БҝОӘ____mgЎӨLЈӯ1Ј¬ИфДі№Өі§ГҝМмЙъІъ1.00 tГҫЈ¬ФтГҝМмРиТӘәЈЛ®өДМе»эОӘ________LЎЈ
ІОҝјҙр°ёЈә(1)Ca(OH)2ЎЎ(2)№эВЛ
(3)СОЛбЎЎMg(OH)2Ј«2HЈ«===Mg2Ј«Ј«2H2O
(4)MgCl2(ИЫИЪ)MgЈ«Cl2Ўь
(5)5.50ЎЎ9.09ЎБ108
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ¶ФКөСйўсЎ«ўфөДКөСйПЦПуФӨІвХэИ·өДКЗЈЁЎЎЎЎЈ©
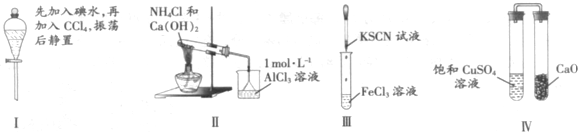
AЈ®КөСй1ЈәТәМе·ЦІгЈ¬ПВІгіКОЮЙ«
BЈ®КөСй2ЈәЙХұӯЦРПИіцПЦ°ЧЙ«іБөнЈ¬әуИЬҪв
CЈ®КөСй3ЈәКФ№ЬЦРСХЙ«ұдОӘәмЙ«
DЈ®КөСй4Јә·ЕТ»¶ОКұјдәуЈ¬ұҘәНCuSO4ИЬТәЦРіцПЦА¶Й«ҫ§Ме
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ