1ЁЂбЁдёЬт ЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ?ЃЉ
AЃЎгУввДМЛђCCl4ПЩЬсШЁЕтЫЎжаЕФЕтЕЅжЪ
BЃЎNaClКЭSiCОЇЬхШмЛЏЪБЃЌПЫЗўСЃзгМфзїгУСІЕФРраЭЯрЭЌ
CЃЎ24Mg32SОЇЬхжаЕчзгзмЪ§гыжазгзмЪ§жЎБШЮЊ1ЉU1
DЃЎH2SКЭSiF4ЗжзгжаИїдзгзюЭтВуЖМТњзу8ЕчзгНсЙЙ
ВЮПМД№АИЃКC
БОЬтНтЮіЃКТд
БОЬтФбЖШЃКМђЕЅ
2ЁЂЬюПеЬт ЃЈ12ЗжЃЉЕквЛЕчРыФмI1ЪЧжИЦјЬЌдзгXЃЈgЃЉДІгкЛљЬЌЪБЃЌЪЇШЅвЛИіЕчзгГЩЮЊЦјЬЌбєРызгXЃЋЃЈgЃЉЫљашвЊЕФФмСПЁЃЯТБэЪЧВПЗждЊЫидзгЕФЕквЛЕчРыФмI1ЃКЃЈЕЅЮЛЃЉ
H
| ?
| ?
| ?
| ?
| ?
| ?
| He
|
1.3120
| ?
| ?
| ?
| ?
| ?
| ?
| 0.3723
|
Li
| Be
| B
| C
| N
| O
| F
| Ne
|
0.5203
| 0.8995
| 0.8001
| 1.0864
| 1.4023
| 1.3140
| 1.6810
| 2.0807
|
Na
| Mg
| Al
| Si
| P
| S
| Cl
| Ar
|
0.4958
| 0.7377
| 0.5776
| 0.7865
| 1.0118
| 0.9996
| 1.2511
| 1.5205
|
K
| Ca
| Ga
| Ge
| As
| Se
| Br
| Kr
|
0.4189
| 0.5898
| 0.5788
| ?
| 0.9440
| 0.9409
| 1.1399
| 1.3507
|
Rb
| Sr
| In
| Sn
| Sb
| Te
| I
| Xe
|
0.4030
| 0.5495
| 0.5583
| 0.7086
| 0.8316
| 0.8693
| 1.0084
| 1.1704
|
Cs
| Ba
| Tl
| Pb
| Bi
| Po
| At
| ?
|
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉДгБэжаЪ§ОнПЩжЊЃЌЭЌвЛжїзхдЊЫидзгЕФЕквЛЕчРыФмI1БфЛЏЙцТЩЪЧЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЃЫЕУїЭЌвЛжїзхдЊЫиЁЁЁЁЁЁЁЁЁЁЁЁЁЁФмСІДгЩЯЕНЯТж№НЅдіЧПЁЃ
ЃЈ2ЃЉДгБэжаЪ§ОндЄВтGeдЊЫиЕквЛЕчРыФмI1ЕФзюаЁЗЖЮЇ?ЁЃ
ЃЈ3ЃЉSiCЪЧдзгОЇЬхЃЌЦфНсЙЙРрЫЦН№ИеЪЏЃЌЮЊCЁЂSiСНдзгвРДЮЯрЭЌХХСаЕФе§ЫФУцЬхПеМфЭјзДНсЙЙЁЃШчЭМЮЊСНИіжааФжиКЯЃЌИїУцЗжБ№ЦНааЕФСНИіе§ЗНЬхЃЌЦфжааФЮЊSiдзгЃЌЪддкаЁе§ЗНЬхЕФЖЅЕуЛГігыSiзюНќЕФCЃЈгУ БэЪОЃЉЕФЮЛжУЃЌдкДѓе§ЗНЬхЕФРтЁЂЖЅЁЂУцЕШДІЛГіЯргІSiЃЈгУ
БэЪОЃЉЕФЮЛжУЃЌдкДѓе§ЗНЬхЕФРтЁЂЖЅЁЂУцЕШДІЛГіЯргІSiЃЈгУ БэЪОЃЉЕФЮЛжУЁЃ
БэЪОЃЉЕФЮЛжУЁЃ
ВЮПМД№АИЃКДгЩЯЕНЯТвРДЮМѕаЁЃЌЪЇЕчзгЁЃ?ЃЈ2ЃЉ0.7086ЁЊ0.7865ЃЈ3ЗжЃЉ
ЃЈ3ЃЉЛЖдЬМдзгЕУ1ЗжЃЌЛЖдЙшдзгЕУ2ЗжЁЃЃЈЙВ3ЗжЃЉ
БОЬтНтЮіЃКЃЈ1ЃЉДгБэжаЪ§ОнПЩжЊЃЌЭЌвЛжїзхдЊЫидзгЕФЕквЛЕчРыФмI1БфЛЏЙцТЩЪЧДгЩЯЕНЯТвРДЮМѕаЁЃЌЫЕУїЪЇЕчзгЕФФмСІж№НЅдіЧПЁЃ
ЃЈ2ЃЉЭЌжїзхздЩЯЖјЯТЕквЛЕчРыФмж№НЅМѕаЁЃЌЫљвдGeЕФЕквЛЕчРыФмДѓаЁЗЖЮЇЪЧ0.7086ЁЊ0.7865ЁЃ
ЃЈ3ЃЉН№ИеЪЏЕФжа4ИіЬМдзгЙЙГЩе§ЫФУцЬхЃЌЫљвдаЁе§ЗНЬхжагыSiзюНќЕФCдзгЮЛгкЫФУцЬхЕФ4ИіЖЅЕуЩЯЃЌД№АИШчЭМЫљЪОЁЃ
БОЬтФбЖШЃКвЛАу
3ЁЂбЁдёЬт ЯТСаЛЏбЇгУгяБэДяе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎS2-ЕФНсЙЙЪОвтЭМЃК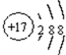
BЃЎHClOЕФНсЙЙЪНЃКH-O-Cl
CЃЎ24CrЕФМђЛЏЕчзгХХВМЪНЃК[Ar]3d44s2
DЃЎH2O2ЕФЕчзгЪНЃКH+[ЃКOЃКOЃК]2-H+
ВЮПМД№АИЃКAЃЎСђРызгКЫЭтга18ИіЕчзгЁЂКЫФкга16ИіжЪзгЃЌСђРызгНсЙЙЪОвтЭМЮЊ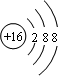 ЃЌЙЪAДэЮѓЃЛ
ЃЌЙЪAДэЮѓЃЛ
BЃЎДЮТШЫсЕФжааФдЊЫиЪЧOдЊЫиЃЌбѕдзгКЭТШдзгЁЂЧтдзгжЎМфЖМЙВгУвЛЖдЕчзгЃЌЫљвдЦфНсЙЙЪНЮЊH-O-ClЃЌЙЪBе§ШЗЃЛ
CЃЎдзгЙьЕРжаЕчзгДІгкАыТњЁЂШЋТњЁЂШЋПеЪБзюЮШЖЈЃЌЫљвдCrдзгЕФМђЛЏЕчзгХХВМЪНЃК[Ar]3d54s1ЃЌЙЪCДэЮѓЃЛ
DЃЎЫЋбѕЫЎЪЧЙВМлЛЏКЯЮяЃЌдзгжЎМфДцдкЙВгУЕчзгЖдЃЌЦфЕчзгЪНЮЊ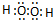 ЃЌЙЪDДэЮѓЃЛ
ЃЌЙЪDДэЮѓЃЛ
ЙЪбЁBЃЎ
БОЬтНтЮіЃК
БОЬтФбЖШЃКвЛАу
4ЁЂЬюПеЬт дЊЫиX ЮЛгкЕкЫФжмЦкЃЌЦфЛљЬЌдзгЕФФкВуЙьЕРШЋВПХХТњЕчзгЃЌЧвзюЭтВуЕчзгЪ§ЮЊ2ЁЃдЊЫиYЛљЬЌдзгЕФ3p ЙьЕРЩЯга4ИіЕчзгЁЃдЊЫиZ ЕФдзгзюЭтВуЕчзгЪ§ЪЧЦфФкВуЕФ3БЖЁЃ
(1)XгыYЫљаЮГЩЛЏКЯЮяОЇЬхЕФОЇАћШчЭМЫљЪОЁЃ

Ђйдк1ИіОЇАћжаЃЌXРызгЕФЪ§ФПЮЊ________ЁЃ
ЂкИУЛЏКЯЮяЕФЛЏбЇЪНЮЊ________ЁЃ
(2)дкYЕФЧтЛЏЮя(H2Y)ЗжзгжаЃЌYдзгЙьЕРЕФдгЛЏРраЭЪЧ________ЁЃ
(3)ZЕФЧтЛЏЮя(H2Z)дкввДМжаЕФШмНтЖШДѓгкH2YЃЌЦфдвђЪЧ________ЁЃ
(4)YгыZПЩаЮГЩYZ42-ЁЃ
ЂйYZ42-ЕФПеМфЙЙаЭЮЊ________(гУЮФзжУшЪі)ЁЃ
ЂкаДГівЛжжгыYZ42-ЛЅЮЊЕШЕчзгЬхЕФЗжзгЕФЛЏбЇЪНЃК________ЁЃ
(5)XЕФТШЛЏЮягыАБЫЎЗДгІПЩаЮГЩХфКЯЮя[X(NH3)4]Cl2ЃЌ1 molИУХфКЯЮяжаКЌгаІвМќЕФЪ§ФПЮЊ________ЁЃ
ВЮПМД№АИЃК(1)Ђй4ЁЁЂкZnS
(2)sp3
(3)ЫЎЗжзггыввДМЗжзгжЎМфаЮГЩЧтМќ
(4)Ђйе§ЫФУцЬхЁЁЂкCCl4ЛђSiCl4ЕШ
(5)16NAЛђ16ЁС6.02ЁС1023Иі
БОЬтНтЮіЃКБОЬтжївЊПМВщдзгНсЙЙгыаджЪжЊЪЖЃЌвтдкПМВщПМЩњЕФПеМфЯыЯѓФмСІМАЖдЮяжЪНсЙЙКЭаджЪЕФРэНтФмСІЁЃXЕФКЫЭтЕчзгХХВМЪНЮЊ1s22s22p63s23p63d104s2ЃЌXЮЊZnЃЛYЕФКЫЭтЕчзгХХВМЪНЮЊ1s22s22p63s23p4ЃЌYЮЊSЃЛИљОнZЕФаХЯЂПЩжЊZЮЊOЁЃ(1)ЂйгЩОЇАћНсЙЙПЩжЊЃЌXЗжБ№ЮЛгкОЇАћЕФЖЅЕуКЭУцаФЃЌИљОнОЇАћжадзгЕФЁАЗжЬЏЗЈЁБПЩМЦЫувЛИіОЇАћжаЕФXдзгЪ§ЮЊЃК8ЁС1/8ЃЋ6ЁС1/2ЃН4ЁЃЂкYдзгШЋВПдкОЇАћжаЃЌЙЪвЛИіОЇАћжаКЌга4ИіYдзгЁЃЙЪИУЛЏКЯЮяЕФЛЏбЇЪНЮЊZnSЁЃ(2)H2SЗжзгжаSдзггаСНЖдГЩМќЕчзгКЭСНЖдЙТЖдЕчзгЃЌЫљвдH2SЗжзгжаSдзгЕФЙьЕРдгЛЏРраЭЮЊsp3дгЛЏЁЃ(3)H2OгыввДМПЩвдаЮГЩЗжзгМфЧтМќЃЌЪЙЕУЫЎгыввДМЛЅШмЃЛЖјH2SгыввДМВЛФмаЮГЩЗжзгМфЧтМќЃЌЙЪH2SдкввДМжаЕФШмНтЖШаЁгкH2OЁЃ(4)ЂйSO42-ЕФжааФдзгSжмЮЇга4ЖдГЩМќЕчзгЃЌаЮГЩвдSЮЊЬхаФЃЌOЮЊЖЅЕуЕФе§ЫФУцЬхНсЙЙЃЛЂкSO42-жаSЁЂOзюЭтВуОљЮЊ6ИіЕчзгЃЌЙЪSO42-жадзгзюЭтВуЙВга32ИіЕчзгЃЛCCl4ЁЂSiCl4жадзгЕФзюЭтВуЕчзгзмЪ§ОљЮЊ4ЃЋ7ЁС4ЃН32ЃЌЙЪSO42-ЁЂCCl4ЁЂSiCl4ЮЊЕШЕчзгЬхЁЃ(5)[Zn(NH3)4]Cl2жа[Zn(NH3)4]2ЃЋгыClЃаЮГЩРызгМќЃЌЖј[Zn(NH3)4]2ЃЋжаКЌга4ИіZnЁЊNМќ(ХфЮЛМќ)КЭ12ИіNЁЊHМќЃЌЙВ16ИіЙВМлЕЅМќЃЌЙЪ1 molИУХфКЯЮяжаКЌга16 mol ІвМќЁЃ
БОЬтФбЖШЃКвЛАу
5ЁЂбЁдёЬт ЛЏбЇПЦбЇашвЊНшжњЛЏбЇзЈгУгябдУшЪіЃЎЯТСагаЙиЛЏбЇгУгяе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
AЃЎCO2ЕФЕчзгЪН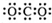
BЃЎввЯЉЗжзгЕФНсЙЙЪН
CЃЎввЫсЕФНсЙЙМђЪНC2H4O2
DЃЎСђдзгЕФдзгНсЙЙЪОвтЭМЃК
ВЮПМД№АИЃКAЃЎCO2ЕФЕчзгЪНЮЊ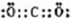 ЃЌЙЪAДэЮѓЃЛ
ЃЌЙЪAДэЮѓЃЛ
BЃЎввЯЉЗжзгЕФНсЙЙЪНЮЊ ЃЌЙЪBе§ШЗЃЛ
ЃЌЙЪBе§ШЗЃЛ
CЃЎввЫсЕФНсЙЙМђЪНЮЊCH3COOHЃЌЙЪCДэЮѓЃЛ
DЃЎСђРызгЕФдзгНсЙЙЪОвтЭМЮЊ ЃЌЖјСђдзгЕФзюЭтВуЕчзгЪ§ЮЊ6ЃЌЙЪDДэЮѓЃЛ
ЃЌЖјСђдзгЕФзюЭтВуЕчзгЪ§ЮЊ6ЃЌЙЪDДэЮѓЃЛ
ЙЪбЁBЃЎ
БОЬтНтЮіЃК
БОЬтФбЖШЃКМђЕЅ