1ЎўСЎФсМв ПВБР·ҙУҰЛщөГөҪөДУР»ъІъОпКЗҙҝҫ»ОпөДКЗ?
[? ]
AЈ®јЧНйУлВИЖш(ОпЦКөДБҝ1©U1)ФЪ№вХХМхјюПВөДИЎҙъ·ҙУҰ?
BЈ®CH3CH2CCl(CH3)2ФЪNaOHҙјИЬТәКЬИИөДПыИҘ·ҙУҰ
CЈ®ұыП©УлВИЖшФЪТ»¶ЁМхјюПВөДјУіЙ·ҙУҰ
DЈ®1-¶ЎП©УлЛ®ФЪТ»¶ЁМхјюПВөДјУіЙ·ҙУҰ
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
2ЎўјтҙрМв (13·Ц)УР»ъОпAУРИзПВЧӘ»Ҝ№ШПөЈ¬ФЪAөДЦКЖЧНјЦРЦКәЙұИЧоҙуЦөОӘ88Ј¬Жд·ЦЧУЦРCЎўHЎўOИэЦЦФӘЛШөДЦКБҝұИОӘ6Јә1Јә4ЗТAІ»ДЬК№Br2өДCCl4ИЬТәНКЙ«Ј»1molB·ҙУҰЙъіЙБЛ2molCЈ®
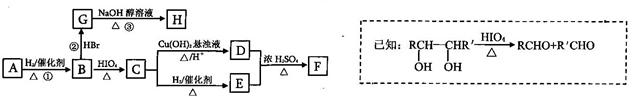
Зл»ШҙрПВБРОКМвЈә
(1)CЎъEөД·ҙУҰАаРНОӘ____________
(2)ИфўЩЎўўЪЎўўЫИэІҪ·ҙУҰөДІъВК·ЦұрОӘ93.0ЈҘЎў81.0ЈҘЎў88.0ЈҘЈ¬ФтУЙAәПіЙHөДЧЬІъВКОӘ____________
(3)AөДҪб№№јтКҪОӘ___________________ЎӨ
(4)РҙіцCУлТш°ұИЬТә·ҙУҰөДАлЧУ·ҪіМКҪОӘ____________________________________
(5)H·ЦЧУЦРЛщУРМјФӯЧУҫщФЪТ»МхЦұПЯЙПЈ¬GЧӘ»ҜОӘHөД»ҜС§·ҪіМКҪОӘ__________________
(6)XКЗAөДТ»ЦЦН¬·ЦТм№№МеЈ¬ЗТЖдәЛҙЕ№ІХсЗвЖЧУР3ёц·еЈ¬·еГж»эЦ®ұИОӘ1Јә1Јә2Ј¬1mol XҝЙФЪHIO4јУИИөДМхјюПВ·ҙУҰЈ¬ІъОпЦ»УР1mol YЈ¬ФтXөДҪб№№јтКҪОӘ____________
ІОҝјҙр°ёЈәЈЁ1Ј©јУіЙ·ҙУҰЈЁ1·ЦЈ©
ЈЁ2Ј©66.3%ЈЁ2·ЦЈ©
ЈЁ3Ј©  ?ЈЁ2·ЦЈ©
?ЈЁ2·ЦЈ©
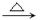 ЈЁ4Ј©CH3CHOЈ«2[Ag(NH3)2]Ј«Ј«2OHЈӯ? CH3COOЈӯЈ«NH4Ј«Ј«2AgЎэЈ«3NH3Ј«H2OЈЁ3·ЦЈ©
ЈЁ4Ј©CH3CHOЈ«2[Ag(NH3)2]Ј«Ј«2OHЈӯ? CH3COOЈӯЈ«NH4Ј«Ј«2AgЎэЈ«3NH3Ј«H2OЈЁ3·ЦЈ©
ЈЁ5Ј©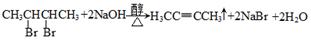 ?ЈЁ3·ЦЈ©
?ЈЁ3·ЦЈ©
ЈЁ6Ј© ?ЈЁ2·ЦЈ©
?ЈЁ2·ЦЈ©
ұҫМвҪвОцЈәёщҫЭМвТвЦӘЈ¬AөДЦКЖЧНјЦРЦКәЙұИЧоҙуЦөОӘ88Ј¬ФтAөДПа¶Ф·ЦЧУЦКБҝОӘ88Ј»·ЦЧУЦРCЎўHЎўOИэЦЦФӘЛШөДЦКБҝұИОӘ6Јә1Јә4Ј¬ҝЙЦӘCЎўHЎўOөДОпЦКөДБҝЦ®ұИОӘ2Јә4Јә1Ј»ЛщТФAөД·ЦЧУКҪОӘC4H8O2Ј¬ёщҫЭ·ЦЧУКҪҝЙЦӘAөДІ»ұҘәН¶ИОӘ1Ј¬ЗТAІ»ДЬК№Br2өДCCl4ИЬТәНКЙ«Ј»ЛщТФAЦРҙжФЪМјСхЛ«јьЈ»УЦ1mol?B·ҙУҰФЪHIO4јУИИөДМхјюПВЙъіЙБЛ2mol?CЈ¬ҪбәПМвёшРЕПўј°ЧӘ»Ҝ№ШПөҝЙНЖЦӘAОӘЈә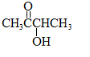 Ј¬BОӘЈә
Ј¬BОӘЈә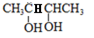 Ј¬CОӘCH3CHOЈ¬DОӘCH3COOHЈ¬EОӘCH3CH2OHЈ¬УЦBУлHBr·ўЙъИЎҙъ·ҙУҰЙъіЙВұҙъМюЈ¬ВұҙъМюФЪNaOHҙјИЬТәЦР·ўЙъПыИҘ·ҙУҰЈ¬ЗТH·ЦЧУЦРЛщУРМјФӯЧУҫщФЪТ»МхЦұПЯЙПЈ¬НЖөГGәНHөДҪб№№·ЦұрОӘЈә
Ј¬CОӘCH3CHOЈ¬DОӘCH3COOHЈ¬EОӘCH3CH2OHЈ¬УЦBУлHBr·ўЙъИЎҙъ·ҙУҰЙъіЙВұҙъМюЈ¬ВұҙъМюФЪNaOHҙјИЬТәЦР·ўЙъПыИҘ·ҙУҰЈ¬ЗТH·ЦЧУЦРЛщУРМјФӯЧУҫщФЪТ»МхЦұПЯЙПЈ¬НЖөГGәНHөДҪб№№·ЦұрОӘЈә Ўў
Ўў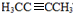 ЎЈЈЁ1Ј©CОӘCH3CHOЈ¬EОӘCH3CH2OHЈ¬CH3CHOҙЯ»ҜјУЗвЙъіЙCH3CH2OHЈ¬ёГ·ҙУҰОӘјУіЙ·ҙУҰЈЁ»т»№Фӯ·ҙУҰЈ©Ј»ЈЁ2Ј©УЙAәПіЙHөДЧЬІъВКУҰОӘўЩЈ¬ўЪЎўўЫИэІҪ·ҙУҰөДІъВКЦ®»эЈ¬ФтУЙAәПіЙHөДЧЬІъВК=93.0%ЎБ81.0%ЎБ88.09%=66.3%Ј»ЈЁ3Ј©ёщҫЭЙПКц·ЦОцЦӘЈ¬ AөДҪб№№ОӘЈә
ЎЈЈЁ1Ј©CОӘCH3CHOЈ¬EОӘCH3CH2OHЈ¬CH3CHOҙЯ»ҜјУЗвЙъіЙCH3CH2OHЈ¬ёГ·ҙУҰОӘјУіЙ·ҙУҰЈЁ»т»№Фӯ·ҙУҰЈ©Ј»ЈЁ2Ј©УЙAәПіЙHөДЧЬІъВКУҰОӘўЩЈ¬ўЪЎўўЫИэІҪ·ҙУҰөДІъВКЦ®»эЈ¬ФтУЙAәПіЙHөДЧЬІъВК=93.0%ЎБ81.0%ЎБ88.09%=66.3%Ј»ЈЁ3Ј©ёщҫЭЙПКц·ЦОцЦӘЈ¬ AөДҪб№№ОӘЈә Ј»ЈЁ4Ј©УЙЙПГжөД·ЦОцҝЙЦӘЈ¬CОӘТТИ©Ј¬ЛьУлТш°ұИЬТә·ҙУҰөДАлЧУ·ҪіМКҪОӘЈәCH3CHO+2[AgЈЁNH3Ј©2]++2OH-?
Ј»ЈЁ4Ј©УЙЙПГжөД·ЦОцҝЙЦӘЈ¬CОӘТТИ©Ј¬ЛьУлТш°ұИЬТә·ҙУҰөДАлЧУ·ҪіМКҪОӘЈәCH3CHO+2[AgЈЁNH3Ј©2]++2OH-? CH3COO-+NH4++2AgЎэ+3NH3+H2OЈ»ЈЁ5Ј©GәНHөДҪб№№·ЦұрОӘЈә
CH3COO-+NH4++2AgЎэ+3NH3+H2OЈ»ЈЁ5Ј©GәНHөДҪб№№·ЦұрОӘЈә Ўў
Ўў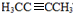 Ј¬GЧӘ»ҜОӘHөД»ҜС§·ҪіМКҪјыҙр°ёЈ»ЈЁ6Ј©XКЗAөДТ»ЦЦН¬·ЦТм№№МеЈ¬ЗТЖдәЛҙЕ№ІХсЗвЖЧУР3ёц·еЈ¬XКЗТ»ёц·ЗіЈ¶ФіЖөДҪб№№Ј¬XЦРБҪёцСхФӯЧУЈ¬·еГж»эЦ®ұИОӘ1Јә1Јә2Ј¬1mol XҝЙФЪHIO4јУИИөДМхјюПВ·ҙУҰЈ¬ІъОпЦ»УР1mol YЈ¬ФтXөДҪб№№јтКҪОӘ
Ј¬GЧӘ»ҜОӘHөД»ҜС§·ҪіМКҪјыҙр°ёЈ»ЈЁ6Ј©XКЗAөДТ»ЦЦН¬·ЦТм№№МеЈ¬ЗТЖдәЛҙЕ№ІХсЗвЖЧУР3ёц·еЈ¬XКЗТ»ёц·ЗіЈ¶ФіЖөДҪб№№Ј¬XЦРБҪёцСхФӯЧУЈ¬·еГж»эЦ®ұИОӘ1Јә1Јә2Ј¬1mol XҝЙФЪHIO4јУИИөДМхјюПВ·ҙУҰЈ¬ІъОпЦ»УР1mol YЈ¬ФтXөДҪб№№јтКҪОӘ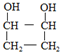 ЎЈ
ЎЈ
ұҫМвДС¶ИЈәТ»°г
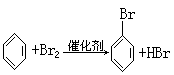
3ЎўМоҝХМв Т»ВұҙъМюRxУлҪрКфДЖЧчУГЈ¬ҝЙТФФцјУМјБҙЦЖёЯј¶МюЈ¬·ҙУҰөД»ҜС§·ҪіМКҪОӘRx+2Na+RЎдxЎъRЎӘRЎд+2NaxЈ¬ЖдЦРxұнКҫВұФӯЧУЈ¬RәНRЎдОӘМю»щЈ¬ҝЙПаН¬ТІҝЙІ»Н¬Ј¬КФТФұҪЎўТТИІЎўBr2ЎўHBrПъОӘЦчТӘФӯБПЈ¬НЁ№эИэІҪ·ҙУҰЦЖИЎ CH=CH2Ј¬ЖдЦЖИЎөД»ҜС§·ҪіМКҪОӘ(1)_______Ј¬(2)______Ј¬(3)_______ЎЈ
CH=CH2Ј¬ЖдЦЖИЎөД»ҜС§·ҪіМКҪОӘ(1)_______Ј¬(2)______Ј¬(3)_______ЎЈ
ІОҝјҙр°ёЈә
(1) 

(3) 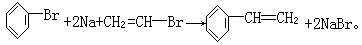
ұҫМвҪвОцЈәКЧПИАыУГұҪәНТТИІөДРФЦКЦЖөГдеұҪәНдеТТП©Ј¬ФЩАыУГЙПКцРЕПўҫНКЗөҪұҪТТП©
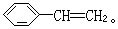
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ЦЖИЎТ»ВИТТНйЈ¬ІЙУГөДЧојС·Ҫ·ЁКЗ
[? ]
AЈ®ТТНйәНВИЖшИЎҙъ·ҙУҰ
BЈ®ТТП©әНВИЖшјУіЙ·ҙУҰ
CЈ®ТТП©әНHClјУіЙ·ҙУҰ
DЈ®ТТП©әНСОЛбЧчУГ
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
5ЎўКөСйМв ЈЁ»ҜС§·ҪіМКҪ2·ЦЈ¬ЖдУаГҝҝХ1·ЦЈ¬№І10·ЦЈ©
ЈЁ1Ј©ФЪNaClИЬТәЦРөОјУAgNO3ИЬТәЈ¬ПЦПуОӘ_____________________________ Ј¬
·ҙУҰөДАлЧУ·ҪіМКҪОӘ:_________________________________________________
ЈЁ2Ј©ФЪCH3CH2CH2ClЦРөОјУAgNO3ИЬТәЈ¬ПЦПуОӘ_______________________________Ј¬
ФӯТтКЗ__________________________
ЈЁ3Ј©ИфПИҪ«CH3CH2CH2ClУлNaOHИЬТә№ІИИЈ¬И»әуУГПхЛбЛб»ҜЈ¬ФЩөОјУAgNO3ИЬТәЈ¬ПЦПуОӘ_______________________________________________________Ј¬·ҙУҰөД»ҜС§·ҪіМКҪОӘ:
___________________________________Ўў______________________________________ЎЈ
ІОҝјҙр°ёЈәЈЁ»ҜС§·ҪіМКҪ2·ЦЈ¬ЖдУаГҝҝХ1·ЦЈ¬№І10·ЦЈ©
ЈЁ1Ј©°ЧЙ«іБөнЈ»Cl- + Ag+ = AgClЎэ
ЈЁ2Ј©ОЮПЦПуЈ¬CH3CH2CH2ClІ»ДЬөзАліцВИАлЧУЈ»
ЈЁ3Ј©°ЧЙ«іБөнЈ»CH3CH2CH2Cl + NaOH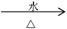 CH3CH2CH2OH + NaCl Ј»
CH3CH2CH2OH + NaCl Ј»
NaCl + AgNO3 =" AgClЎэ+" NaNO3
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ