1ЎўСЎФсМв ЙиnAОӘ°ў·ьЩӨөВВЮіЈКэөДКэЦөЈ¬ПВБРЛө·ЁХэИ·өДКЗ
AЈ®іЈОВПВЈ¬23g NO2ә¬УРnAёцСхФӯЧУ
BЈ®1L0.1mol?L-1өД°ұЛ®ә¬УР0.1nAёцOHЁD
CЈ®іЈОВіЈС№ПВЈ¬22.4LCCl4ә¬УРёцnAёцCCl4·ЦЧУ
DЈ®1molFe2+ УлЧгБҝөДH2O2ИЬТә·ҙУҰЈ¬ЧӘТЖ2nAёцөзЧУ
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈә
ХэИ·ҙр°ёЈәA
ұҫМвҝјІмТФОпЦКөДБҝОӘәЛРДөДУР№ШјЖЛгәНЕР¶ПЎЈNO2өДДҰ¶ыЦКБҝКЗ46g/molЈ¬ЛщТФ23g NO2өДОпЦКөДБҝКЗ0.5molЈ¬ә¬УРСхФӯЧУөДОпЦКөДБҝКЗ0.5molЎБ2ЈҪ1molЈ¬јҙә¬УРNAёцСхФӯЧУЈ¬AХэИ·Ј»NH3ЎӨH2OКфУЪИхөзҪвЦКЈ¬ФЪИЬТәЦРІҝ·ЦөзАлЈ¬ТтҙЛ1L 0.1molЎӨLЈӯ1өД°ұЛ®І»ҝЙДЬөзАліц0.1molOHЈӯЈ¬BІ»ХэИ·Ј»іЈОВіЈС№ПВЈ¬CCl4КЗТәМеЈ¬ТтҙЛІ»ККУГУЪЖшМеөДДҰ¶ыМе»эЈ¬јҙ22.4 L CCl4І»КЗ1molЈ¬CІ»ХэИ·Ј»Fe2+ ұ»Сх»ҜЈ¬ІъОпКЗFe3+ Ј¬ТтҙЛ1 mol Fe2+ УлЧгБҝөДH2O2ИЬТә·ҙУҰЈ¬ЧӘТЖNAёцөзЧУЈ¬DТІІ»ХэИ·ЎЈ
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ұкЧјЧҙҝцПВЈ¬ИфvLЗвЖшә¬УРөДЗв·ЦЧУКэОӘNЈ¬Фт°ў·ьјУөВВЮіЈКэҝЙұнКҫОӘ?
AЈ®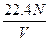 ?molЎӘ1? BЈ®
?molЎӘ1? BЈ®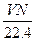 molЎӘ1?CЈ®
molЎӘ1?CЈ®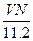 ?molЎӘ1? DЈ®
?molЎӘ1? DЈ®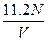 ?molЎӘ1
?molЎӘ1
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ
3ЎўСЎФсМв ФЪұкЧјЧҙҝцПВЈ¬іЖБҝТ»ёцідВъВИЖшөДИЭЖчЦКБҝОӘ74.6ҝЛЈ¬ИфёДідөӘЖшЈ¬ЖдЦКБҝОӘ66ҝЛЈ¬ФтИЭЖчөДИЭ»эОӘЈЁЎЎЎЎЈ©
AЈ®11.2L
BЈ®5.6L
CЈ®4.48L
DЈ®2.24L
ІОҝјҙр°ёЈәјЩЙиЖшМеөДОпЦКөДБҝОӘnmolЈ¬ИЭЖчөДЦКБҝОӘmgЈ¬ФтЈә
mg+nmolЎБ71g/mol=74.6g
mg+nmolЎБ28g/mol=66g
БӘБў·ҪіМЈ¬ҪвөГn=0.2mol
ЖшМеөДМе»эОӘV=0.2molЎБ22.4L/mol=4.48LЈ®
ЛщТФИЭЖчөДИЭ»эОӘ4.48LЈ®
№КСЎCЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ТФПВҫц¶ЁОпЦКРФЦКөДТтЛШИ«ГжЎўХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®ФӘЛШөД»ҜС§РФЦКЈәФӘЛШФӯЧУөДЧоНвІгөзЧУКэ
BЈ®KNO3өДИЬҪв¶ИЈәОВ¶И
CЈ®·ЦЧУјдЧчУГБҰЈәПа¶Ф·ЦЧУЦКБҝ
DЈ®ЖшМеДҰ¶ыМе»эЈәОВ¶ИЈ¬ГЬ¶И
ІОҝјҙр°ёЈәAЈ®У°ПмФӘЛШ»ҜС§РФЦКөДЦчТӘТтЛШКЗЧоНвІгөзЧУКэЈ¬ө«ФӯЧУ°лҫ¶І»Н¬Ј¬ОпЦКөДРФЦКТІІ»Н¬Ј¬ҫЯУРПаЛЖРФәНөЭұдРФөД№жВЙЈ¬№КAҙнОуЈ»
BЈ®ОВ¶ИІ»Н¬Ј¬ОпЦКөДИЬҪв¶ИІ»Н¬Ј¬ФтОВ¶ИКЗУ°ПмИЬҪв¶ИөДЦчТӘТтЛШЈ¬№КBХэИ·Ј»
CЈ®·ЦЧУјдЧчУГБҰөДҙуРЎИЎҫцУЪПа¶Ф·ЦЧУЦКБҝәН·ЦЧУҪб№№Ј¬·ЦЧУҪб№№ПаН¬КұЈ¬·ЦЧУјдЧчУГБҰЦчТӘИЎҫцУЪПа¶Ф·ЦЧУЦКБҝҙуРЎЈ¬№КCҙнОуЈ»
DЈ®У°ПмЖшМеДҰ¶ыМе»эөДТтЛШКЗОВ¶ИәНС№ЗҝЈ¬¶шІ»КЗГЬ¶ИЈ¬№КDҙнОуЈ®
№КСЎBЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ФЪПаН¬өДОВ¶ИәНС№ЗҝПВЈ¬4ёцИЭЖчЦР·ЦұрЧ°УР4ЦЦЖшМеЎЈТСЦӘёчИЭЖчЦРөДЖшМеәНИЭЖчөДИЭ»э·ЦұрКЗЈәaЎўCO2Јә100mLЈ»bЎўO2Јә200mLЈ»cЎўN2Јә400mLЈ»dЎўCH4Јә600mLЎЈФт4ёцИЭЖчЦРЖшМеөДЦКБҝУЙҙуөҪРЎөДЛіРтКЗ?
[? ]
AЈ®cЈҫdЈҫbЈҫa
BЈ®bЈҫaЈҫdЈҫc
CЈ®aЈҫbЈҫcЈҫd
DЈ®dЈҫcЈҫaЈҫb
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ