1ЎўјтҙрМв №ӨТөЙъІъПхЛбп§өДБчіМНјИзНјЈә
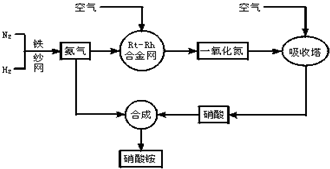
Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©РҙіцПхЛбп§ФЪ№ӨЕ©ТөЙъІъЦРөДЦШТӘЧчУГ______ЈЁИОРҙТ»МхЈ©
ЈЁ2Ј©ТСЦӘN2ЈЁgЈ©+3H2ЈЁgЈ©ЁT2NH3ЈЁgЈ©Ј¬ЎчH=-92kJ?mol-1Ј®Зл»ШҙрЈә
ўЩФЪ500ЎжЎў200atmәНМъҙЯ»ҜМхјюПВПтТ»ГЬұХИЭЖчЦРідИл1molN2әН3molH2Ј¬ід·Ц·ҙУҰәуЈ¬·ЕіцөДИИБҝ______ЈЁМоЎ°ЈјЎұЎ°ЈҫЎұЎ°=ЎұЈ©92.4kJЈ¬АнУЙКЗ______Ј®
ўЪОӘУРР§МбёЯЗвЖшөДЧӘ»ҜВКЈ¬КөјКЙъІъЦРТЛІЙИЎөДҙлК©УР______
AЈ®ҪөөНОВ¶И?BЈ®ЧоККәПҙЯ»ҜјБ»оРФөДККөұёЯОВ?CЈ®ФцҙуС№Зҝ
DЈ®ҪөөНС№Зҝ?EЈ®Сӯ»·АыУГәНІ»¶ПІ№ідөӘЖш?FЈ®ј°КұТЖіц°ұ
ЈЁ3Ј©ТСЦӘІ¬ооәПҪрНшОҙФӨИИТІ»б·ўИИЈ®Рҙіц°ұҙЯ»ҜСх»ҜөД»ҜС§·ҪіМКҪЈә______Ј¬ёГ·ҙУҰөД»ҜС§ЖҪәвіЈКэұнҙпКҪK=______Ј¬өұОВ¶ИЙэёЯКұЈ¬KЦө______ЈЁФцҙуЎўјхРЎЎўОЮУ°ПмЈ©Ј¬ЖдФӯТтКЗ______Ј®
ЈЁ4Ј©ФЪТ»¶ЁОВ¶ИәНС№ЗҝөДГЬұХИЭЖчЦРЈ¬Ҫ«ЖҪҫщКҪБҝОӘ8.5өДH2әНN2»мәПЈ¬өұёГ·ҙУҰҙпөҪЖҪәвКұЈ¬ІвіцЖҪәв»мәПЖшөДЖҪҫщКҪБҝОӘ10Ј¬ҙЛКұN2өДЧӘ»ҜВКОӘ______Ј®
ІОҝјҙр°ёЈәЈЁ1Ј©Ттп§СОөДЦчТӘУГНҫКЗУГЧчөӘ·КЈ¬№Кҙр°ёОӘЈәҝЙЧцөӘ·КөИЈ»
? ЈЁ2Ј©ўЩТтОӘҝЙДж·ҙУҰІ»ҝЙДЬНкИ«ҪшРРөҪөЧЈ¬·ЕіцөДИИБҝұИНкИ«·ҙУҰЙЩЈ¬
№Кҙр°ёОӘЈәЈјЈ¬ФЪ1atmәН298KМхјюПВЈ¬1molөӘЖшәН3molЗвЖшНкИ«·ҙУҰЙъіЙ2mol°ұЖшЈ¬·Еіц92.4kJИИБҝЈ¬ёГ·ҙУҰОӘҝЙДж·ҙУҰЈ¬І»ҝЙДЬҪшРРНкИ«Ј¬УЦТтОӘ·ҙУҰОВ¶ИОӘ500ЎжЈ¬ЛщТФ·ЕіцөДИИБҝРЎУЪ92.4kJЈ»?
? ?ўЪТтФцҙу·ҙУҰОпөДЕЁ¶ИЈ¬јхЙЩЙъіЙОпөДЕЁ¶ИЈ¬ФцҙуС№ЗҝЈ¬ҪөөНОВ¶И№ӨТөәПіЙ°ұ·ҙУҰПтХэ·ҙУҰ·ҪПтТЖ¶ҜЈ¬ЗвЖшөДЧӘ»ҜВКМбёЯЈ¬ө«ФЪКөјКЙъІъЦРІ»ДЬУГөНОВЈ¬ТтОӘОВ¶ИөН»ҜС§·ҙУҰЛЩВКВэЈ¬№Кҙр°ёОӘЈәCEFЈ»
?ЈЁ3Ј©ТтОӘNH3ҫЯУР»№ФӯРФЈ¬ДЬұ»СхЖшСх»ҜЈә4NH3+5O2?јУИИ?.?4NO+6H2OЈ¬
? ?ёГ·ҙУҰөДЖҪәвіЈКэK=C5(NO)?C6(H2O)C4(NH3)?C5(O2)?
? ?Тт№ӨТөәПіЙ°ұ·ҙУҰКЗ·ЕИИ·ҙУҰЈ¬ЙэёЯОВ¶ИЈ¬ЖҪәвПтДж·ҙУҰ·ҪПтТЖ¶ҜЈ¬»ҜС§ЖҪәвіЈКэјхРЎЈ¬
? №Кҙр°ёОӘЈә4NH3+5O2?јУИИ?.?4NO+6H2OЈ¬C5(NO)?C6(H2O)C4(NH3)?C5(O2)Ј¬јхРЎЈ¬ТтОӘ°ұөДҙЯ»ҜСх»Ҝ·ҙУҰКЗ·ЕИИ·ҙУҰЈ¬ЛщТФЙэёЯОВ¶ИЈ¬K»бјхРЎЈ»
?ЈЁ4Ј©К®ЧЦҪ»Іж·ЁЈәЖҪҫщПа¶Ф·ЦЧУЦКБҝОӘ8.5өДH2әНN2ОпЦКөДБҝұИ=ЈЁ28-8.5Ј©ЈәЈЁ8.5-2Ј©=3Јә1
? ЖҪәвИэІҝЗъЈәN2 +3H2 =2NH3
? Жр? 1? 3? ?0
?·ҙ? ?x? ?3x? ?2x
? Д©? ?1-x? 3-3x? ?2x?
? ЖҪәв»мәПЖшөДЖҪҫщПа¶Ф·ЦЧУЦКБҝОӘ10Ј¬¶ФУҰөДПа¶Ф·ЦЧУЦКБҝЈә28ЎБ1+2ЎБ3(1-x)+(3-3x)+2x=10
? x=0.3
?ФтH2ЧӘ»ҜВКОӘ3ЎБ0.33ЎБ100%=30%
?№Кҙр°ёОӘЈә30%
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўјтҙрМв ЈЁ1Ј©Ҫ«І»Н¬БҝөДCOЈЁgЈ©әНH2OЈЁgЈ©·ЦұрНЁИлМе»э2LөДәгИЭГЬұХИЭЖчЦРЈ¬ҪшРР·ҙУҰCOЈЁgЈ©+H2OЈЁgЈ©?CO2ЈЁgЈ©+H2ЈЁgЈ©Ј¬өГөҪИзПВБҪЧйКэҫЭЈә
| КөСйЧй | ОВ¶ИЎж | ЖрКјБҝ/mol | ЖҪәвБҝ/mol | ҙпөҪЖҪәвЛщРиКұјд/min
CO
H2O
H2
CO
1
650
4
2
1.6
2.4
6
2
900
2
1
0.4
1.6
3
|
ўЩКөСй1ЦРТФvЈЁCO2Ј©ұнКҫөД·ҙУҰЛЩВКОӘ______ЈЁөЪ¶юО»РЎКэЈ©
ўЪёГ·ҙУҰОӘ______ЈЁМоЎ°ОьИИЎұ»тЎ°·ЕИИЎұЈ©·ҙУҰЈ®
ўЫЗуКөСй2өДЖҪіЈіЈКэKЈ¬ТӘЗуРҙіцјЖЛг№эіМЈ¬Ҫб№ыИЎ¶юО»РЎКэ
ЈЁ2Ј©ТСЦӘФЪіЈОВіЈС№ПВЈәўЩ2CH3OHЈЁ1Ј©+3O2ЈЁgЈ©=2CO2ЈЁgЈ©+4H2OЈЁ1Ј©ЎчH=-1451.6kJ/mol
ўЪ2COЈЁgЈ©+O2ЈЁgЈ©=2CO2ЈЁgЈ©ЎчH=-566.0kJ/mol
РҙіцјЧҙјІ»НкИ«ИјЙХЙъіЙCOәНТәМ¬Л®өДИИ»ҜС§·ҪіМКҪ______
ЈЁ3Ј©јЧҙјәНСхЖшНкИ«ИјЙХөД·ҙУҰҝЙТФЙијЖОӘИјБПөзіШЈ¬Ч°ЦГИзНјЈ¬ёГөзіШНЁ№эK2CO3ИЬТәОьКХ·ҙУҰЙъөДCO2Ј®Фтёәј«өДөзј«·ҙУҰОӘ______Ј®
ЈЁ4Ј©CaCO3өДKSP=2.8ЎБ10-9Ј®Ҫ«өИМе»эCaCl2ИЬТәУлNa2CO3ИЬТә»мәПЈ¬ИфNa2CO3ИЬТәөДЕЁ¶ИОӘ2ЎБ10-4mol/LЈ¬ФтЙъіЙіБөнЛщРиёГCaCl2ИЬТәөДЧоРЎЕЁ¶ИОӘ______Ј®
ІОҝјҙр°ёЈәЈЁ1Ј©ўЩЎўУЙұнЦРКэҫЭҝЙЦӘЈ¬COөДОпЦКөДБҝұд»ҜБҝОӘ4mol-2.4mol=1.6molЈ¬vЈЁCOЈ©=1.6mol2L6min=215mol/ЈЁL?minЈ©Ј¬ЛЩВКЦ®ұИөИУЪ»ҜС§јЖБҝКэЦ®ұИЈ¬№КvЈЁCO2Ј©=vЈЁCOЈ©=215mol/ЈЁL?minЈ©=0.13mol/ЈЁL?minЈ©Ј¬
№Кҙр°ёОӘЈә0.13mol/ЈЁL?minЈ©Ј»
ўЪЎўКөСй1ЦРCOөДЧӘ»ҜВКОӘ4mol-2.4mol4molЎБ100%=40%Ј¬КөСй2ЦРCOөДЧӘ»ҜВКОӘ2mol-1.6mol2molЎБ100%=20%Ј¬ФтКөСй1өДЧӘ»ҜВКҙуУЪКөСй2Ј¬ФтЛөГчОВ¶ИЙэёЯЖҪәвПтДж·ҙУҰ·ҪПтТЖ¶ҜЈ¬Хэ·ҙУҰ·ЕИИЈ¬
№Кҙр°ёОӘЈә·ЕИИЈ»
ўЫЎўЖҪәвКұCOөДОпЦКөДБҝОӘ1.6molЈ¬ФтЈә
? COЈЁgЈ©+H2OЈЁgЈ©?CO2ЈЁgЈ©+H2ЈЁgЈ©Ј¬
ҝӘКјЈЁmolЈ©Јә2? 1?0? ?0
ұд»ҜЈЁmolЈ©Јә0.4? 0.4? 0.4? 0.4
ЖҪәвЈЁmolЈ©Јә1.6? 0.6? 0.4? 0.4
ёГ·ҙУҰЗ°әуЖшМеМе»эІ»ұдЈ¬№КАыУГОпЦКөДБҝҙъМжЕЁ¶ИјЖЛгЖҪәвіЈКэЈ¬№К900ЎжКұёГ·ҙУҰЖҪәвіЈКэk=0.4ЎБ0.41.6ЎБ0.6=0.17Ј¬
№Кҙр°ёОӘЈә0.17Ј»
ЈЁ2Ј©ТСЦӘЈәўЩ2CH3OHЈЁ1Ј©+3O2ЈЁgЈ©=2CO2ЈЁgЈ©+4H2OЈЁ1Ј©ЎчH=-1451.6kJ/mol
ўЪ2COЈЁgЈ©+O2ЈЁgЈ©=2CO2ЈЁgЈ©ЎчH=-566.0kJ/mol
ёщҫЭёЗЛ№¶ЁВЙЈ¬ўЩ-ўЪөГ2CH3OHЈЁ1Ј©+2O2ЈЁgЈ©=2COЈЁgЈ©+4H2OЈЁ1Ј©Ј¬№КЎчH=ЈЁ-1451.6kJ/molЈ©-ЈЁ-566.0kJ/molЈ©=
885.6kJ/molЈ¬јҙCH3OHЈЁlЈ©+O2ЈЁgЈ©=COЈЁgЈ©+2H2OЈЁlЈ©ЎчH=-442.8kJ/molЈ¬
№Кҙр°ёОӘЈәCH3OHЈЁlЈ©+O2ЈЁgЈ©=COЈЁgЈ©+2H2OЈЁlЈ©ЎчH=-442.8kJ/molЈ»
ЈЁ3Ј©ЧЬөДөзіШ·ҙУҰКҪОӘЈә2CH3OH+3O2+2CO32-=4HCO3-+2H2OЈ¬јЧҙј·ўЙъСх»Ҝ·ҙУҰЈ¬ФЪёәј«·ЕөзЈ¬СхЖш·ўЙъ»№Фӯ·ҙУҰЈ¬ФЪХэј«·ЕөзЈ¬Хэј«өзј«·ҙУҰКҪОӘ3O2+12e-+12HCO3-=12CO32-+6H2OЈ¬ЧЬ·ҙУҰКЗјхИҘХэј«·ҙУҰКҪҝЙөГёәј«өзј«·ҙУҰКҪОӘЈә
2CH3OH+14CO32-+4H2O-12e-=16HCO3-Ј¬јҙCH3OH+7CO32-+2H2O-6e-=8HCO3-Ј¬
№Кҙр°ёОӘЈәCH3OH+7CO32-+2H2O-6e-=8HCO3-Ј»
Хэј«өзј«·ҙУҰКҪОӘ3O2+12e-+6H2O=12OH-Ј¬
ЈЁ4Ј©УЙ»мәПәуМјЛбёщЕЁ¶ИОӘ1ЎБ10-4mol/LЈ¬»мәПәуК№МјЛбёщіБөнЈ¬
РиТӘёЖАлЧУөДЧоРЎЕЁ¶ИОӘ2.8ЎБ10-91ЎБ10-4mol/L=2.8ЎБ10-5mol/LЈ¬
ФӯВИ»ҜёЖЕЁ¶ИОӘ»мәПәуёЖАлЧУЕЁ¶ИөД2ұ¶Ј¬№КФӯВИ»ҜёЖөДЕЁ¶ИОӘ2ЎБ2.8ЎБ10-5mol/L=5.6ЎБ10-5mol/LЈ¬
№Кҙр°ёОӘЈә2.8ЎБ10-5mol/LЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
3ЎўјЖЛгМв ТТҙјЖыУНКЗұ»№г·әК№УГөДРВРНЗеҪаИјБПЈ¬№ӨТөЙъІъТТҙјөДТ»ЦЦ·ҙУҰФӯАнОӘЈә
2CO(g) + 4H2(g) CH3CH2OH(g) + H2O(g) ЎчH =" ЎӘ256.1" kJЎӨmolЈӯ1
CH3CH2OH(g) + H2O(g) ЎчH =" ЎӘ256.1" kJЎӨmolЈӯ1
ТСЦӘЈәCO(g) + H2O(g) CO2(g)+H2(g) ЎчH=" ЎӘ41.2" kJЎӨmolЈӯ1
CO2(g)+H2(g) ЎчH=" ЎӘ41.2" kJЎӨmolЈӯ1
ЈЁ1Ј©ТФCO2(g)УлH2(g)ОӘФӯБПТІҝЙәПіЙТТҙјЈ¬ЖдИИ»ҜС§·ҪіМКҪИзПВЈә
2CO2(g) +6H2(g) CH3CH2OH(g) +3H2O(g) ЎчH = ЎЈ
CH3CH2OH(g) +3H2O(g) ЎчH = ЎЈ
ЈЁ2Ј©ЖыіөК№УГТТҙјЖыУНІўІ»ДЬјхЙЩNOxөДЕЕ·ЕЈ¬ХвК№NOxөДУРР§ПыіэіЙОӘ»·ұЈБмУтөДЦШТӘҝОМвЎЈ
ўЩДіСРҫҝРЎЧйФЪКөСйКТТФAg
ІОҝјҙр°ёЈә
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәА§ДС
4ЎўјЖЛгМв ЈЁ10·ЦЈ©ЈЁ1Ј©УГCH4ҙЯ»Ҝ»№ФӯNOxҝЙТФПыіэөӘСх»ҜОпөДОЫИҫЎЈАэИзЈә
CH4(g)+4NO2(g)ЈҪ4NO(g)Ј«CO2(g)+2H2O(g)ЎЎҰӨЈИЈҪЈӯ574 kJЎӨmol-1
CH4(g)+4NO(g)ЈҪ2N2(g)Ј«CO2(g)+2H2O(g)ЎЎҰӨЈИЈҪЈӯ1160 kJЎӨmol-1
ИфУГұкЧјЧҙҝцПВ4.48ЈМCH4»№ФӯNO2ЦБN2Ј¬Хыёц№эіМЦРЧӘТЖөДөзЧУЧЬКэОӘЎЎЎЎЎЎЎЎЈЁ°ў·ьЩӨөВВЮіЈКэУГNAұнКҫЈ©Ј¬·ЕіцөДИИБҝОӘЎЎЎЎЎЎЎЎЎЎ kJЎЈ
ЈЁ2Ј©ТСЦӘЈәC3H8(g ) ==== CH4(g)Ј«HCЎФCH(g)Ј«H2(g) ЎчH1ЈҪ156.6kJЎӨmol-1
CH3CHЈҪCH2(g) ==== CH4(g)Ј«HCЎФCH(g) ЎчH2ЈҪ32.4kJЎӨmol-1
ФтПаН¬МхјюПВЈ¬·ҙУҰC3H8(g)====CH3CHЈҪCH2 (g)Ј«H2(g)өДЎчHЈҪ kJЎӨmol-1ЎЈ
(3)јЧНйФЪёЯОВПВУлЛ®ХфЖш·ҙУҰ·ҙУҰ·ҪіМКҪОӘЈәCH4(g)Ј«H2O(g)ЈҪCO(g)Ј«3H2(g)ЎЈІҝ·ЦОпЦКөДИјЙХИИКэҫЭИзУТұнЈәТСЦӘ1mol H2O(g)ЧӘұдОӘ1mol H2O(l)Кұ·Еіц44.0 kJИИБҝЎЈРҙіцCH4әНH2OФЪёЯОВПВ·ҙУҰөДИИ»ҜС§·ҪіМКҪ ЎЈ
Оп ЦК
| ИјЙХИИЈЁkJЎӨmolЈӯ1Ј©
|
H2(g)
| Јӯ285.8
|
CO(g)
| Јӯ283.0
|
CH4(g)
| Јӯ890.3
|
(4)УРИЛЙиПлС°ЗуәПККөДҙЯ»ҜјБәНөзј«ІДБПЈ¬ТФN2ЎўH2ОӘөзј«·ҙУҰОпЈ¬ТФHClЈӯNH4ClОӘөзҪвЦКИЬТәЦЖИЎРВРНИјБПөзіШЎЈЗлРҙіцёГөзіШөДХэј«·ҙУҰКҪ ЎЈ
ІОҝјҙр°ёЈәЈЁ10·ЦЈ© ЈЁ1Ј© 1Ўў6NA 173Ўў4 ЈЁ2Ј© +124Ўў2
ЈЁ3Ј©CH4(g) + H2O(g) ="=" CO(g) + 3H2(g) ?H= --1420Ўў7 kJ/mol
(4) N2 + 6e + 8H+ ="=" 2NH4+
ұҫМвҪвОцЈәЈЁ1Ј©ёщҫЭёЗЛ№¶ЁВЙҝЙЦӘЈ¬ўЩЈ«ўЪјҙөГөҪCH4(g)Ј«2NO2(g)ЈҪN2(g)Ј«CO2(g)Ј«2H2O(g)ЎЎҰӨHЈҪЈӯ867 kJЎӨmolЈӯ1ЎЈұкЧјЧҙҝцПВ4.48 L CH4өДОпЦКөДБҝОӘ0.2 molЈ¬К§ИҘ0.2molЎБ8ЈҪ1.6molөзЧУЈ¬·ЕіцөДИИБҝОӘ0.2 molЎБ867 kJЎӨmolЈӯ1ЈҪ173.4 kJЎЈ
ЈЁ2Ј©Н¬СщёщҫЭёЗЛ№¶ЁВЙҝЙЦӘЈ¬ўЩЈӯўЪјҙөГөҪC3H8(g)====CH3CHЈҪCH2 (g)Ј«H2(g)Ј¬ЛщТФёГ·ҙУҰөДЎчHЈҪЈ«156.6kJЎӨmol-1Јӯ32.4kJЎӨmol-1ЈҪЈ«124.2kJ/molЎЈ
ЈЁ3Ј©ёщҫЭОпЦКөДИјЙХИИҝЙЦӘЈ¬ўЩH2(g)+1/2O2(g)ЈҪH2O(l)ЎЎҰӨЈИЈҪЈӯ285.8 kJЎӨmol-1ЎўўЪCO(g)+1/2O2(g)ЈҪCO2(g)ЎЎҰӨЈИЈҪЈӯ283.0 kJЎӨmol-1ЎўўЫCH4(g)+2O2(g)ЈҪCO2(g)+2H2O(l)ЎЎҰӨЈИЈҪЈӯ890.3 kJЎӨmol-1ЎўўЬ H2O(g)ЈҪH2O(l) ЎчHЈҪЈӯ44.0 kJ/molЎЈЛщТФёщҫЭёЗЛ№¶ЁВЙҝЙЦӘЈ¬ўЫЈ«ўЬЈӯўЩЎБ3ЈӯўЪјҙөГөҪCH4(g) + H2O(g) ="=" CO(g) + 3H2(g) ?H= --1420Ўў7 kJ/molЎЈ
ЈЁ4Ј©ФӯөзіШЦРХэј«өГөҪөзЧУЈ¬ЛщТФөӘЖшФЪХэј«өГөҪөзЧУЎЈУЦТтОӘИЬТәПФЛбРФЈ¬ЛщТФХэј«өзј«·ҙУҰКҪКЗN2 + 6e + 8H+ ="=" 2NH4+ЎЈ
ҝјөгЈәҝјІй·ҙУҰИИөДјЖЛгЎўИИ»ҜС§·ҪіМКҪөДКйРҙТФј°өзј«·ҙУҰКҪөДКйРҙөИ
өгЖАЈәКйРҙИИ»ҜС§·ҪіМКҪТФј°·ҙУҰИИөДјЖЛгЦРЈ¬ёЗЛ№¶ЁВЙУРЧЕ№г·әөДУҰУГЈ¬РиТӘКмБ·ХЖОХІўҪбәПУР№ШКэС§ЦӘК¶Бй»оФЛУГЎЈ
ұҫМвДС¶ИЈәА§ДС
5ЎўСЎФсМв °ЧБЧУлСхҝЙ·ўЙъИзПВ·ҙУҰЈәP4+5O2= P4O10ЎЈТСЦӘ¶ПБСПВБР»ҜС§јьРиТӘОьКХөДДЬБҝ·ЦұрОӘЈә PЈӯPЈәa kJЎӨmol-1ЎўPЈӯOЈәb kJЎӨmol-1ЎўP=OЈәc kJЎӨmol-1ЎўO=OЈәd kJЎӨmol-1Ј¬ёщҫЭНјКҫөД·ЦЧУҪб№№әНУР№ШКэҫЭ№АЛгёГ·ҙУҰөД HЈ¬ЖдЦРХэИ·өДКЗ( )
HЈ¬ЖдЦРХэИ·өДКЗ( )
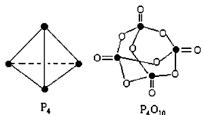
AЈ®(6a+5dЈӯ4cЈӯ12b) kJЎӨmol-1
BЈ®(4c+12bЈӯ6aЈӯ5d) kJЎӨmol-1
CЈ®(4c+12bЈӯ4aЈӯ5d) kJЎӨmol-1
DЈ®(4a+5dЈӯ4cЈӯ12b) kJЎӨmol-1