1ЎўјтҙрМв УЙЗвЖшәНСхЖш·ҙУҰЙъіЙ1molЛ®ХфЖш·ЕИИ241.8kJЈ¬РҙіцёГ·ҙУҰөДИИ»ҜС§·ҪіМКҪЈә______Ј®
Иф1gЛ®ХфЖшЧӘ»ҜіЙТәМ¬Л®·ЕИИ2.444kJЈ¬Фт·ҙУҰH2ЈЁgЈ©+
O2ЈЁgЈ©ЁTH2OЈЁlЈ©ЎчH=______kJ?mol-1Ј®ЗвЖшөДИјЙХИИОӘ______kJ?mol-1Ј®
ІОҝјҙр°ёЈәЗвЖшәНСхЖш·ҙУҰЙъіЙ1molЛ®ХфЖш·ЕИИ241.8kJЈ¬ёГ·ҙУҰөДИИ»ҜС§·ҪіМКҪОӘЈәH2ЈЁgЈ©+12O2ЈЁgЈ©=H2OЈЁgЈ©ЎчH=-241.8kJ/molЈ»
1gЛ®ХфЖшЧӘ»ҜіЙТәМ¬Л®·ЕИИ2.444kJЈ¬№К18gЛ®ХфЖшЧӘ»ҜіЙТәМ¬Л®·ЕіцИИБҝ2.444kJЎБ18=44kJЈ¬№К·ҙУҰH2ЈЁgЈ©+12O2ЈЁgЈ©ЁTH2OЈЁlЈ©өД·ҙУҰИИЎчH=-ЈЁ241.8kJ/mol+44kJ/molЈ©=-285.8kJ/molЈ¬№КЗвЖшөДИјЙХИИОӘ285.8kJ/molЈ»
№Кҙр°ёОӘЈәH2ЈЁgЈ©+12O2ЈЁgЈ©=H2OЈЁgЈ©ЎчH=-241.8kJ/molЈ»-285.5Ј»285.5Ј®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ·ҙУҰ2C+O2=2COөДДЬБҝұд»ҜИзПВНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗ
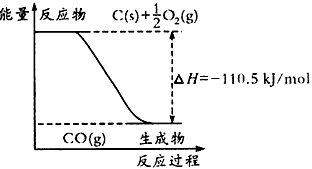
[? ]
AЈ®12g C(s)УлТ»¶ЁБҝO2(g)·ҙУҰЙъіЙ14g CO(g)Ј¬·ЕіцөДИИБҝОӘ110.5 kJ
BЈ®2 mol C(s)УлЧгБҝO2(g)·ҙУҰЙъіЙCO2(g)Ј¬·ЕіцөДИИБҝҙуУЪ221 kJ/mol
CЈ®ёГ·ҙУҰөДИИ»ҜС§·ҪіМКҪКЗЈә2C(s) +O2(g)=2CO(g) ЎчH= -221kJ/mol
DЈ®МјөДИјЙХИИОӘ110. 5 kJ/mol
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
3ЎўСЎФсМв јЧНйКЗТ»ЦЦёЯР§ЗеҪаөДРВДЬФҙЈ¬0Ј®25molјЧНйНкИ«ИјЙХЙъіЙТәМ¬Л®Кұ·Еіц222Ј®5KJИИБҝЈ¬ФтПВБРИИ»ҜС§·ҪіМКҪЦРХэИ·өДКЗ
[? ]
AЈ®2CH4(g) + 4O2(g) == 2CO2(g) + 4H2O(l) ҰӨH == +890 kJЎӨmol-1
BЈ®CH4(g) + 2O2(g) == CO2(g) +2H2O(l) ҰӨH == +890 kJЎӨmol-1
CЈ®CH4(g) + 2O2(g) == CO2(g) + 2H2O(l) ҰӨH == -890 kJЎӨmol-1
DЈ®2CH4(g) + 4O2(g) == 2CO2(g) +4H2O(l) ҰӨH == -890 kJЎӨmol-1
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
4ЎўМоҝХМв РҙіцПВБРИИ»ҜС§·ҪіМКҪЈә
(1)2mol Cu(s)УлККБҝO2(g)·ҙУҰЙъіЙCuO(s)Ј¬·Еіц 314 kJИИБҝ____________Ј»
(2)1mol N2(g)УлККБҝO2(g)·ҙУҰЙъіЙNO(g)Ј¬РиОьКХ 68 kJөДИИБҝ______________Ј»
(3)ұкЧјЧҙҝцПВЈ¬12 gМҝ·ЫФЪСхЖшЦРІ»НкИ«ИјЙХЙъіЙТ»Сх»ҜМјЈ¬·Еіц110. 35 kJөДИИБҝ________________ЎЈ
ІОҝјҙр°ёЈә(1) 2Cu (s) +O2 (g) =2CuO(s) ЎчH = - 314kJЎӨmol-1
(2) N2 (g) +O2 ( g) =2NO( g) ЎчH = +68kJЎӨmol-1
(3) 2C(s) +O2(g)=2CO(g) ЎчH=-220.7kJЎӨmol-1
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўМоҝХМв УГCH4ҙЯ»Ҝ»№ФӯNOxҝЙТФПыіэөӘСх»ҜОпөДОЫИҫЈ®АэИзЈә
CH4ЈЁgЈ©+4NO2ЈЁgЈ©ЁT4NOЈЁgЈ©+CO2ЈЁgЈ©+2H2OЈЁgЈ©ЎчH=-574kJ?mol-1
CH4ЈЁgЈ©+4NOЈЁgЈ©ЁT2N2ЈЁgЈ©+CO2ЈЁgЈ©+2H2OЈЁgЈ©ЎчH=-1160kJ?mol-1
ИфУГұкЧјЧҙҝцПВ4.48LCH4»№ФӯNO2ЦБN2Ј¬Хыёц№эіМЦРЧӘТЖөДөзЧУЧЬКэОӘ______Ј¬РҙіцЙПКц·ҙУҰөДИИ»ҜС§·ҪіМКҪЈә______Ј®
ІОҝјҙр°ёЈәТСЦӘўЩCH4ЈЁgЈ©+4NO2ЈЁgЈ©ЁT4NOЈЁgЈ©+CO2ЈЁgЈ©+2H2OЈЁgЈ©ЎчH=-574kJ?mol-1Ј¬ўЪCH4ЈЁgЈ©+4NOЈЁgЈ©ЁT2N2ЈЁgЈ©+CO2ЈЁgЈ©+2H2OЈЁgЈ©ЎчH=-1 160kJ?mol-1Ј¬
АыУГёЗЛ№¶ЁВЙҪ«ўЩ+ўЪ2ҝЙөГCH4ЈЁgЈ©+2NO2ЈЁgЈ©ЁTN2ЈЁgЈ©+CO2ЈЁgЈ©+2H2OЈЁgЈ©Ј¬ЎчH=-574+11602kJ?mol-1=867kJ?mol-1Ј¬
nЈЁCH4Ј©=0.2molЈ¬Хыёц№эіМЦРЧӘТЖөДөзЧУЧЬКэОӘ0.2molЎБ8NA=1.6NA=9.6ЎБ1023Ј¬
№Кҙр°ёОӘЈә9.6ЎБ1023»т1.6NAЈ»CH4ЈЁgЈ©+2NO2ЈЁgЈ©ЁTN2ЈЁgЈ©+CO2ЈЁgЈ©+2H2OЈЁgЈ©ЎчH=-867kJ?mol-1Ј®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г