1ЎўСЎФсМв Діә¬ёх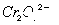 ·ПЛ®БтЛбСЗМъп§
·ПЛ®БтЛбСЗМъп§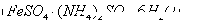 ҙҰАнЈ¬·ҙУҰЦРМъФӘЛШәНёхФӘЛШНкИ«ЧӘ»ҜОӘіБөнЎЈёГіБөнҫӯёЙФпәуөГөҪn mol
ҙҰАнЈ¬·ҙУҰЦРМъФӘЛШәНёхФӘЛШНкИ«ЧӘ»ҜОӘіБөнЎЈёГіБөнҫӯёЙФпәуөГөҪn mol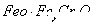 ?ЎЈІ»ҝјВЗҙҰАн№эіМЦРөДКөјКЛрәДЈ¬ПВБРРрКцҙнОуөДКЗ
?ЎЈІ»ҝјВЗҙҰАн№эіМЦРөДКөјКЛрәДЈ¬ПВБРРрКцҙнОуөДКЗ
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈә
ҙр°ёЈәA
Cr2O72ЁDҫЯУРЗҝСх»ҜРФЈ¬FeSO4ЎӨ(NH4)2SO4ЎӨ6H2OҫЯУРЗҝ»№ФӯРФЈ¬¶юХЯ·ўЙъСх»Ҝ»№Фӯ·ҙУҰЈ¬Fe2Ј«ұ»Сх»ҜіЙFe3Ј«Ј¬Cr2O72ЁDЦРЈ«6јЫCrұ»»№ФӯіЙЈ«3јЫCrЎЈёГ·ҙУҰЦРЈ¬FeК§өзЧУөДОпЦКөДБҝөИУЪCrөГөзЧУөДОпЦКөДБҝЈ¬ФтУРny molЈҪ3nx molЈ¬јҙ3xЈҪyЎЈҫЭCrЎўFe ФӯЧУКШәгҝЙЦӘЈ¬ЙъіЙn mol FeOЎӨFeyCrxO3КұЈ¬ПыәД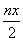 ?mol Cr2O72ЁDЈ¬ПыәДn(yЈ«1) molБтЛбСЗМъп§Ј¬·ҙУҰЦРЧӘТЖөзЧУөДОпЦКөДБҝОӘ
?mol Cr2O72ЁDЈ¬ПыәДn(yЈ«1) molБтЛбСЗМъп§Ј¬·ҙУҰЦРЧӘТЖөзЧУөДОпЦКөДБҝОӘ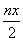 ?molЎБ6ЈҪ3nx molЈ¬УЦЦӘ3xЈҪyФтПыәДБтЛбСЗМъп§өДОпЦКөДБҝОӘn(3xЈ«1) molЎЈ№КAҙнОуЈ¬BЎўCЎўDХэИ·ЎЈ
?molЎБ6ЈҪ3nx molЈ¬УЦЦӘ3xЈҪyФтПыәДБтЛбСЗМъп§өДОпЦКөДБҝОӘn(3xЈ«1) molЎЈ№КAҙнОуЈ¬BЎўCЎўDХэИ·ЎЈ
ұҫМвДС¶ИЈәТ»°г
2ЎўМоҝХМв ёхөД»ҜәПОпУР¶ҫЈ¬УЙУЪ+6јЫCrөДЗҝСх»ҜРФЈ¬Жд¶ҫРФКЗ+3јЫCr¶ҫРФөД100ұ¶ЎЈТтҙЛЈ¬ұШРл¶Фә¬ёхөД·ПЛ®ҪшРРҙҰАнЎЈДҝЗ°СРҫҝәНІЙУГөДҙҰАн·Ҫ·ЁЦчТӘУРЈә
·Ҫ·ЁТ»Ўў»№Фӯ·ЁЈә
ЈЁўсЈ©ФЪЛбРФҪйЦКЦРУГFeSO4ЎўNaHSO3өИҪ«+6јЫCr»№ФӯіЙ+3јЫCrЎЈҫЯМеБчіМИзПВЈә

Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ЙПКцБчіМўЩ·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪКЗ?ЎЈ?
ЈЁ2Ј©ИфФЪўЩ К№FeSO4ККөұ№эБҝЎўҝХЖшККБҝЈ¬ҝЙІъЙъҫЯУРҙЕРФЎўЧйіЙАаЛЖУЪМъСхМеЈЁFe3O4»тFeOЎӨFe2O3Ј©өДёҙәПСх»ҜОпЈЁ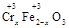 ЎӨy
ЎӨy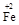 OЈ©(ЧўЈәXҝЙТФКЗ·ЦКэ)Ј¬ұд·ПОӘұҰЎЈФтҝШЦЖҝХЖшККБҝөДДҝөДКЗ?Ј¬К№МъСхМе·ЦАліцАҙҪПјтұгөД·Ҫ·ЁКЗ?ЎЈјЩЙиҙҰАнә¬1 mol Cr2O72ЈӯөД·ПЛ®ЦБЙЩРиТӘјУИл10mol FeSO4ЎӨ7H2OФтёҙәПСх»ҜОпЈЁ
OЈ©(ЧўЈәXҝЙТФКЗ·ЦКэ)Ј¬ұд·ПОӘұҰЎЈФтҝШЦЖҝХЖшККБҝөДДҝөДКЗ?Ј¬К№МъСхМе·ЦАліцАҙҪПјтұгөД·Ҫ·ЁКЗ?ЎЈјЩЙиҙҰАнә¬1 mol Cr2O72ЈӯөД·ПЛ®ЦБЙЩРиТӘјУИл10mol FeSO4ЎӨ7H2OФтёҙәПСх»ҜОпЈЁ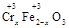 ЎӨy
ЎӨy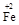 OЈ©»ҜС§КҪКЗ?ЎЈ
OЈ©»ҜС§КҪКЗ?ЎЈ
ЈЁўтЈ©СРҫҝ·ўПЦЈ¬УГМъ·ЫЧч»№ФӯјБІ»ҪцҝЙТФіэИҘCr6+Ј¬»№ДЬіэИҘ·ПЛ®ЦРөДЙЩБҝMn2+Ј¬ПЦСРҫҝМъРјУГБҝәНpHЦө¶Ф·ПЛ®ЦРёхЎўГМИҘіэВКөДУ°ПмЈ¬
ЈЁ3Ј©ИЎ100mL·ПЛ®УЪ250 mLИэҪЗЖҝЦРЈ¬өчҪЪpHЦөөҪ№ж¶ЁЦөЈ¬·ЦұрјУИлІ»Н¬БҝөД·ПМъРјЈ®өГөҪМъРјУГБҝ¶ФёхәНГМИҘіэВКөДУ°ПмИзПВНј1ЛщКҫЎЈФтФЪpHТ»¶ЁКұЈ¬·ПЛ®ЦРМъРјУГБҝОӘ?КұГМЎўёхИҘіэВКЧоәГ
ЈЁ4Ј©ИЎ100mL·ПЛ®УЪ250 mLИэҪЗЖҝЦРЈ¬јУИл№ж¶ЁБҝөДМъ·ЫЈ¬өчіЙІ»Н¬өДpHЦөЎЈөГөҪpHЦө¶ФёхәНГМИҘіэВКөДУ°ПмИзПВНј2ЛщКҫЎЈФтФЪМъРјУГБҝТ»¶ЁКұЈ¬·ПЛ®pH=??КұГМЎўёхИҘіэВКЧоәГ


·Ҫ·Ё¶юЎўөзҪв·ЁЈәҪ«ә¬+6јЫCrөД·ПЛ®·ЕИлөзҪвІЫДЪЈ¬УГМъЧчСфј«Ј¬јУИлККБҝөДВИ»ҜДЖҪшРРөзҪвЈәСфј«ЗшЙъіЙөДFe2+әНCr2O72-·ўЙъ·ҙУҰЈ¬ЙъіЙөДFe3+әНCr3К®ФЪТхј«ЗшУлOHТ»ҪбәПЙъіЙFeЈЁOHЈ©3әНCrЈЁOHЈ©3іБөніэИҘЎЈ
ЈЁ5Ј©РҙіцҙЛТхј«·ҙУҰөДөзј«·ҪіМКҪ?ЎЈ
ЈЁ6Ј©ПЦУГЙП·ЁҙҰАн1ЎБ104 Lә¬ёхЈЁ+6јЫЈ©78 mg / LөД·ПЛ®Ј¬өзҪвКұСфј«ОпЦКПыәДөДЦКБҝЦБЙЩОӘ________kgЎЈ
ІОҝјҙр°ёЈәЈЁ16·ЦЈ©ЈЁ1Ј©Cr2O72-Ј«14H+Ј«6Fe2+=6Fe3+Ј«2Cr3К®Ј«7H2OЈЁ2·ЦЈ©Ј©

ЈЁ2Ј©К№ИЬТәЦРөДұИАэЗЎөұЈЁҙр·АЦ№Fe2+ұ»СхЖш№э¶ИСх»ҜҝЙТФёш·ЦЈ©ЈЁ2·ЦЈ©?
УГҙЕМъОьТэЈЁ2·ЦЈ©? Cr0.5Fe1.5O3ЎӨFeOЈЁ2·ЦЈ©
ЈЁ3Ј©15% ЈЁ2·ЦЈ©ЈЁ4Ј© 4 ЈЁ2·ЦЈ©ЈЁ5Ј©2H2OЈ«2eЈӯЈҪH2ЎьЈ«2OHЈӯЎЈ ЈЁ2·ЦЈ© ЈЁ6Ј© 2Ј®52 ЈЁ2·ЦЈ©
ұҫМвҪвОцЈәЈЁ1Ј©Cr2O72ЈӯУРҪПЗҝСх»ҜРФЈ¬FeSO4ЦРFe2+УРТ»¶ЁөД»№ФӯРФЈ¬ФЪЛбРФҪйЦКЦР·ўЙъСх»Ҝ»№Фӯ·ҙУҰЈ¬УЙКөСйБчіМҝЙЦӘЈ¬өЪўЩІҪ·ҙУҰЦРCr2O72ЈӯФЪЛбРФМхјюПВҪ«Fe2+Сх»ҜОӘFe3+Ј¬ЧФЙнұ»»№ФӯОӘCr3+Ј¬ёщҫЭКШәгФӘЛШКШәгј°ЛщҙҰ»·ҫіҝЙЦӘЈ¬»№УҰУРЛ®ЙъіЙЈ¬·ҙУҰАлЧУ·ҪіМКҪОӘCr2O72Јӯ+14H++6Fe2+=2Cr3++6Fe3++7H2OЎЈ
ЈЁ2Ј©ҝХЖшЦРә¬УРСхЖшЈ¬Из№ыҝХЖш№эБҝЈ¬ФтFe2+ұ»СхЖш№э¶ИСх»ҜЈ¬К№ИЬТәЦРFe2Ј«әНFe3Ј«өДұИАэІ»ЗЎөұЎЈУЙУЪМъСхМеҫЯУРҙЕРФЈ¬ЛщТФК№МъСхМе·ЦАліцАҙҪПјтұгөД·Ҫ·ЁКЗУГҙЕМъОьТэЎЈёщҫЭ·ҙУҰөДАлЧУ·ҪіМКҪҝЙЦӘЈ¬1mol Cr2O72ЈӯДЬСх»Ҝ6molСЗМъАлЧУЈ¬Н¬КұЙъіЙ2mol Cr3К®Ј¬¶шКЈУаСЗМъАлЧУөДОпЦКөДБҝКЗ4molЈ¬ЛщТФёщҫЭФӯЧУКШәгәН»ҜәПјЫҙъКэәНОӘ0ҝЙЦӘЈ¬ Ўў3xЈ«6Јӯ3xЈ«2yЈҪ8Ј¬ҪвөГxЈҪ0.5ЎўyЈҪ1Ј¬ЛщТФёҙәПСх»ҜОпөД»ҜС§КҪКЗCr0.5Fe1.5O3ЎӨFeOЎЈ
Ўў3xЈ«6Јӯ3xЈ«2yЈҪ8Ј¬ҪвөГxЈҪ0.5ЎўyЈҪ1Ј¬ЛщТФёҙәПСх»ҜОпөД»ҜС§КҪКЗCr0.5Fe1.5O3ЎӨFeOЎЈ
ЈЁ3Ј©ёщҫЭНј1ЦӘЈ¬өұМъУГБҝЦрҪҘФцҙуКұЈ¬АлЧУөДИҘіэВКПИФцҙуәујхРЎЈ¬өұ·ПЛ®ЦРМъРјУГБҝОӘ15%КұГМЎўёхИҘіэВКЧоәГЎЈ
ЈЁ4Ј©ёщҫЭНј2ЦӘЈ¬өұИЬТәөДpHЦөЦрҪҘФцҙуКұЈ¬ГМАлЧУөДИҘіэВКПИјхРЎәуФцҙуЈ¬ёхөДИҘіэВКПИФцҙуәујхРЎЈ¬өұpH=4КұГМЎўёхИҘіэВКЧоәГЎЈ
ЈЁ5Ј©өзҪвіШЦРТхј«өГөҪөзЧУЈ¬ФтТхј«КЗЛ®өзАліцөДЗвАлЧУөГөҪөзЧУЈ¬ЛщТФТхј«·ҙУҰөДөзј«·ҪіМКҪ2H2OЈ«2eЈӯЈҪH2ЎьЈ«2OHЈӯЎЈ
ЈЁ6Ј©1ЎБ104 Lә¬ёхЈЁ+6јЫЈ©78 mg / LөД·ПЛ®ЦРёхФӘЛШөДЦКБҝКЗ78 mg / LЎБ10000ЈҪ780000mgЈҪ780gЈ¬ОпЦКөДБҝКЗ780gЎВ52g/molЈҪ15molЎЈФЪ·ҙУҰЦРөГөҪ15moЎБ3ЈҪ45molөзЧУЈ¬ТтҙЛёщҫЭөзЧУөДөГК§КШәгҝЙЦӘЈ¬ІОјУ·ҙУҰөДСЗМъАлЧУКЗ45molЈ¬ЛщТФСфј«ПыәДМъөДЦКБҝЦБЙЩКЗ45molЎБ56g/molЈҪ2520gЈҪ2.52kgЎЈ
өгЖАЈәұҫМвТФ№ӨТө·ПЛ®ҙҰАнОӘФШМеЈ¬ҝјІйСх»Ҝ»№Фӯ·ҙУҰЎўАлЧУ·ҙУҰЎўөзј«·ҙУҰКҪөДКйРҙТФј°УР№ШјЖЛгөИЈ¬ДС¶ИЦРөИЈ¬№ШјьёщҫЭКөСйБчіМАыУГСх»Ҝ»№Фӯ·ҙУҰЕР¶П·ўЙъөДАлЧУ·ҙУҰЈ¬КЗ¶ФС§ЙъЧЫәПДЬБҰөДҝјІйЈ®КЗТ»өАІ»ҙнөДДЬБҰҝјІйМвЈ¬УРАыУЪЕаСшС§ЙъөДВЯјӯНЖАнДЬБҰәН·ўЙўЛјО¬ДЬБҰЈ¬ТІУРЦъУЪЕаСшС§ЙъөД»·ҫіұЈ»ӨТвК¶Ј¬МбЙэС§ЙъөДС§ҝЖЛШСшЎЈ
ұҫМвДС¶ИЈәА§ДС
3ЎўМоҝХМв ЈЁ15·ЦЈ©ДіҙэІвТәҝЙДЬУРFe2+ЎўFe3+ЎўAg+ЎўBa2+ЎўAl3+ЎўCa2+ЎўNH4+өИАлЧУЈ¬ҪшРРБЛПВКцКөСйЈЁЛщјУЛбЎўјоЎўNH3Л®ЎўBr2Л®¶јКЗ№эБҝөДЈ©ЎЈ

ёщҫЭКөСйҪб№ыЈә
ЈЁ1Ј©ЕР¶ЁҙэІвТәЦРУРОЮBa2+ЎўCa2+АлЧУЈ¬ІўРҙіцАнУЙЎЈҙр_________ЎЈ
ЈЁ2Ј©Рҙіц»ҜС§КҪЈәіБөнA_________Ј»іБөнD_________Ј»іБөнE_________ЎЈ
ЈЁ3Ј©РҙіцПВБРАлЧУ·ҙУҰ·ҪіМКҪЈә
деЛ®Сх»ҜИЬТәAЦРөДДіАлЧУЈә_________ЎЈ
іБөнCјУИлЧгБҝNaOHИЬТәәуЖдЦРТ»ЦЦіБөнИЬҪвЈә_________ЎЈ
ІОҝјҙр°ёЈәЈЁ1Ј©ә¬УРBa2+ЎўCa2+АлЧУЦРөДТ»ЦЦ»тБҪЦЦЈ¬ТтОӘBaSO4І»ИЬУЪЛ®Ј¬CaSO4ОўИЬУЪЛ®ЈЁ2Ј© AЈәAgClЈ»? DЈәFeЈЁOHЈ©3Ј»? E ЈәAlЈЁOHЈ©3
ЈЁ3Ј© 2Fe2++Br2=2Fe3++2Br-?Ј»AlЈЁOHЈ©3+OHЎӘ=AlO2ЎӘ+2H2O
ұҫМвҪвОцЈәіБөнAҝЙЕР¶ПОӘAgClЈ¬ЛщТФAg+ТСҙУИЬТәЦРіэөфЈ¬ИЬТәAЦРИфә¬Fe2+ТСұ»деЛ®Сх»ҜОӘFe3+ЎЈУЙУЪBaSO4ДСИЬУЪЛ®Ј¬CaSO4ОўИЬУЪЛ®Ј¬ҝЙЕР¶ПіБөнBҝЙДЬКЗ¶юХЯЦ®Т»Ј¬ТІҝЙДЬКЗЛьГЗөД»мәПОпЎЈAlЈЁOHЈ©3Ј¬FeЈЁOHЈ©3¶јДСИЬУЪЛ®Ј¬іБөнCҝЙДЬКЗ¶юХЯЦ®Т»Ј¬»тКЗЛьГЗөД»мәПОпЎЈИфіБөнCЦРУРAlЈЁOHЈ©3Ј¬јУИл№эБҝNaOHИЬТәәуЙъіЙNaAlO2ҪшИлИЬТәDЈ¬УГ№эБҝNaOHИЬТәіБөнCәуЈ¬ИФКЈУаіБөнDЈ¬ҝЙЕР¶ПіБөнDКЗFeЈЁOHЈ©3ЎЈHAlO2КЗұИH2CO3ёьИхөДЛбЈ¬CO2№эБҝКЗТЧіцҙнЦ®ҙҰІъОпКЗHCO3ЎӘЈ¬AlЈЁOHЈ©3ҫНКЗіБөнEЎЈ
AlO2ЎӘ+CO2+2H2O=AlЈЁOHЈ©3Ўэ+HCO3ЎӘ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗ
A.ЗвСх»ҜГҫИЬУЪПЎБтЛбЈәMg(OH)2+2H+ЈҪMg2++2H2O
B.іОЗеКҜ»ТЛ®АҙОьКХВИЖшЈәCl2+OH-ЈҪCl-+ClO-+H+
C.№эБҝөДМъУлЕЁHNO3·ҙУҰЈә3Fe+8H++2NO3-ЈҪ3Fe3++2NOЎь+4H2O
D.ФЪМјЛбЗвёЖИЬТәЦРјУИлЙЩБҝҝБРФјШИЬТәЈәCa2++2HCO3-+2OH-ЈҪCaCO3Ўэ+CO32-+2H2O
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
5ЎўСЎФсМв ПВБРёчЧйАлЧУЈ¬ФЪөОјУКҜИпКФТәәу»бұдА¶өДИЬТәЦРДЬҙуБҝ№ІҙжЈ¬ЗТИЬТәОӘОЮЙ«НёГчөДАлЧУЧйКЗ
A.K+ЎўMnO4-ЎўCl-ЎўSO42-
B.Na+ЎўNO3-ЎўHCO3-ЎўCl-
C.Na+ЎўH+ЎўNO3-ЎўSO42-
D.Na+ЎўNO3-ЎўSO42-ЎўCl-
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә·ЦОцЈәөОјУКҜИпКФТәәу»бұдА¶өДИЬТәЦРУРҙуБҝөДOH-Ј¬АлЧУЦ®јдІ»ДЬҪбәПЙъіЙЛ®ЎўЖшМеЎўіБөнөИЈ¬ФтАлЧУДЬҙуБҝ№ІҙжЈ¬ІўЧўТвАлЧУөДСХЙ«АҙҪвҙрЈ®
ҪвҙрЈәAЈ®ёГЧйАлЧУЦ®јдІ»·ҙУҰЈ¬ДЬ№ІҙжЈ¬ЗТMnO4-ОӘЧПЙ«Ј¬УлОЮЙ«ИЬТәІ»·ыЈ¬№КAІ»СЎЈ»
BЈ®ТтHCO3-ЎўOH-ДЬҪбәПЙъіЙЛ®әНМјЛбёщАлЧУЈ¬ФтІ»ДЬ№ІҙжЈ¬№КBІ»СЎЈ»
CЈ®ТтOH-ЎўH+ДЬҪбәПЙъіЙЛ®Ј¬ФтІ»ДЬ№ІҙжЈ¬№КCІ»СЎЈ»
DЈ®ТтёГЧйАлЧУФЪјоИЬТәЦРІ»·ҙУҰЈ¬ФтДЬ№ІҙжЈ¬ЗТАлЧУҫщОӘОЮЙ«Ј¬№КDСЎЈ»
№КСЎDЈ®
өгЖАЈәұҫМвҝјІйАлЧУөД№ІҙжОКМвЈ¬КмПӨёҙ·ЦҪв·ҙУҰ·ўЙъөДМхјюј°АлЧУЦ®јдөД·ҙУҰјҙҝЙҪвҙрЈ¬ДС¶ИІ»ҙуЈ®
ұҫМвДС¶ИЈәјтөҘ