1¡¢Ñ¡Ôñ̀â ijÈÜ̉ºÖĐ¼ÓÈëÂÁ·ÛÓĐÇâÆø²úÉú£¬¸ĂÈÜ̉ºÖĐ̉»¶¨ÄÜ´óÁ¿¹²´æµÄÀë×Ó×éÊÇ (? )
A£®Na+¡¢NO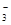 ¡¢Cl-¡¢SO
¡¢Cl-¡¢SO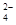
B£®Na+¡¢K+¡¢Ba2+¡¢HCO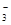
C£®Fe2+¡¢NH4+¡¢NO3?¡¢Cl-
D£®Na+¡¢K+¡¢Cl-¡¢SO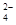
2¡¢Ñ¡Ôñ̀â ÏÂÁи÷½MÀë×ÓÔÚÖ¸¶¨µÄÈÜ̉ºÖĐ̉»¶¨ÄÜ´óÁ¿¹²´æµÄÊÇ(¡¡¡¡? )
A£® c(H+)/c(OH-)=1012µÄÈÜ̉ºÖĐ:NH4+¡¢K+¡¢HCO3-¡¢NO3-
B£® c(OH-)=0.1mol/LµÄÈÜ̉ºÖĐ£ºCO32-¡¢Cl-¡¢F-¡¢K+
C£®º¬´óÁ¿Al3+µÄÈÜ̉ºÖĐ: K+¡¢Na+¡¢SO32-¡¢ClO-
D£®¼×»ù³È³Ê»ÆÉ«µÄÈÜ̉ºÖĐ£ºI-¡¢Cl-¡¢NO3-¡¢Na+
3¡¢Ñ¡Ôñ̀â ÏÂÁĐÄÜÔÚÈÜ̉ºÖĐ´óÁ¿¹²´æµÄ̉»×éÀë×ÓÊÇ
A£®Cu2+¡¢SO42-¡¢OH£¡¢K+?
B£®Na¡¢CO32-¡¢H+¡¢Cl-
C£®H+¡¢Cl-¡¢NO3-¡¢Ba2+
D£®Ca2+¡¢H+¡¢CO32-¡¢NO3-
4¡¢Ñ¡Ôñ̀â ÏÂÁи÷×éÖеÄÀë×Ó£¬ÄÜÔÚÈÜ̉ºÖĐ´óÁ¿¹²´æµÄÊÇ£¨¡¡¡¡£©
A£®Mg2+¡¢Ba2+¡¢OH-¡¢NO3-
B£®Ba2+¡¢Na+¡¢CO32-¡¢OH-
C£®K+¡¢Mg2+¡¢NO3-¡¢Cl-
D£®H+¡¢K+¡¢CO32-¡¢SO42-
5¡¢Ñ¡Ôñ̀â ÏÂÁи÷×éÀë×ÓÔÚÖ¸¶¨ÈÜ̉ºÖĐÄÜ´óÁ¿¹²´æµÄÊÇ£¨ £©
A£®ÔÚÎ̃É«ÈÜ̉ºÖĐ£º Na+¡¢Cu2+¡¢NO3£ ¡¢MnO4£
B£®ÔÚº¬ÓĐHCO3£µÄÈÜ̉ºÖĐ£ºH+¡¢K+¡¢SO42£¡¢Cl£
C£®pH=1µÄÈÜ̉ºÖĐ£ºNa+¡¢ K+¡¢AlO2£¡¢SO42£
D£®³£ÎÂÏ£¬Ë®µçÀëµÄc(H+)=10£12mol¡¤L£1µÄÈÜ̉ºÖĐ£ºK+¡¢Ba2+¡¢NO3£¡¢Cl£