1ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®ҙОВИЛбёЖИЬТәЦРНЁИлЙЩБҝSO2ЖшМеЈәCa2++2ClO-+SO2+H2OЁTCaSO3Ўэ+2HClO
BЈ®МјЛбЗвёЖИЬТәЦРјУИл№эБҝөДNaOHИЬТәЈәCa2++HCO3-+OH-=CaCO3Ўэ+H2O
CЈ®BaЈЁOHЈ©2ИЬТәјУИлNaHSO4ИЬТәЦБёХәГіБөнНкИ«ЈәBa2++OH-+H++SO42-=BaSO4Ўэ+H2O
DЈ®ПтFeSO4ИЬТәЦРөОјУH2O2ИЬТәЈәFe2++H2O2+2H+=Fe3++2H2O
ІОҝјҙр°ёЈәAЎўЙъіЙОпЦРөДҙОВИЛбҫЯУРСх»ҜРФЈ¬ДЬҪ«СЗБтЛбёЖСх»ҜЈ¬¶юХЯІ»ДЬН¬КұҙжФЪЈ¬№КAҙнОуЈ»
BЎўМјЛбЗвёЖИЬТәЦРјУИл№эБҝөДNaOHИЬТәКұЈ¬МјЛбЗвёЖЦРөДёЖАлЧУәНМјЛбЗвёщ°ҙ1Јә2өДЧйіЙұИҪшРР·ҙУҰЈ¬јҙCa2++2HCO3-+2OH-=CaCO3Ўэ+H2O+CO32-Ј¬№КBҙнОуЈ»
CЎўBaЈЁOHЈ©2ИЬТәјУИлNaHSO4ИЬТәЦБЗвСх»ҜұөЦРөДұөАлЧУЗЎәГіБөнКұЈ¬ЗвСхёщ»бКЈУаЈ¬јҙBa2++OH-+H++SO42-=BaSO4Ўэ+H2OЈ¬№КCХэИ·Ј»
DЎўАлЧУ·ҙУҰЧсСӯөзәЙКШәгЈ¬Л«СхЛ®Сх»ҜСЗМъАлЧУөД·ҙУҰОӘЈә2Fe2++H2O2+2H+=2Fe3++2H2OЈ¬№КDҙнОуЈ®
№КСЎCЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
2ЎўСЎФсМв ПВБРКЗДіН¬С§¶ФПаУҰ·ҙУҰөДАлЧУ·ҪіМКҪЛщЧчөДЖАјЫЈ¬ЖдЦРЖАјЫәПАнөДКЗЈЁ?Ј©
ұаәЕ
| »ҜС§·ҙУҰ
| АлЧУ·ҪіМКҪ
| ЖАјЫ
|
A
| МјЛбёЖУлҙЧЛб·ҙУҰ
| CO32ЎӘ+2CH3COOH=" " CO2Ўь+ H2O+2CH3COOЎӘ
| ҙнОуЈ¬МјЛбёЖКЗ
ИхөзҪвЦКЈ¬І»УҰ
РҙіЙАлЧУРОКҪ
|
B
| ПтAlCl3ИЬТәЦРјУИл№эБҝөД°ұЛ®
| Al3+ + 3NH3ЎӨH2O = AlЈЁOHЈ©3Ўэ + 3NH4+
| ҙнОуЈ¬јо№эБҝЈ¬
УҰЙъіЙAlO2ЎӘ
|
C
| NaHCO3өДЛ®Ҫв
| HCO3ЎӘ+H2O CO32ЎӘ+H3O+ CO32ЎӘ+H3O+
| ХэИ·
|
D
| өИОпЦКөДБҝөД
FeBr2әНCl2·ҙУҰ
| 2Fe2+ + 2BrЎӘ+ 2Cl2 = 2Fe3+ + Br2 + 4ClЎӘ
| ХэИ·
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәAАлЧУ·ҪіМТ§САЗРіЭј°ЖАјЫҫщҙнОуЈ¬МјЛбёЖІ»І»ИЬОпЈ¬І»ДЬІрҪвОӘАлЧУЈ»BЈәАлЧУ·ҪіМКҪХэИ·Ј¬ЖАјЫҙнОуЈ»CЈәМвДҝёшіцөДКЗNaHCO3өДөзАл·ҪіМКҪЈ¬ЖдЛ®Ҫв·ҪіМКҪОӘЈәHCO3ЎӘ+H2O H2CO32ЎӘ+OHЎӘ H2CO32ЎӘ+OHЎӘ
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ПВБРёч·ҙУҰөДАлЧУ·ҪіМКҪЦР,КйРҙХэИ·өДКЗ(? )
AЈ® NaOHИЬТәУлHCl·ҙУҰ: H+ + OH- = H2O
BЈ® FeCl3әНFe·ҙУҰ: Fe3+ +Fe = 2Fe2+
CЈ®БтЛбәНЗвСх»Ҝұө·ҙУҰ: H+ + SO42- + Ba2+ +OH- = H2O + BaSO4Ўэ
DЈ®Сх»ҜНӯәНПЎСОЛб·ҙУҰ: CuO + 2H+ = Cu2+ + H2O
ІОҝјҙр°ёЈәAD
ұҫМвҪвОцЈәA ¶Ф
B ҙн өзәЙІ»КШәг
C ҙн 2H+ + SO42- + Ba2+ +2OH- = 2H2O + BaSO4Ўэ
D ¶Ф
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ПВБРАл·ҪіМКҪКйРҙХэИ·өДКЗЈЁ?Ј©
AЈ®КҜ»ТИйУлNa2CO3ИЬТә»мәПЈәCa2Ј«Ј«CO32ЈӯЈҪCaCO3
BЈ®NH4HSO3ИЬТәУлЧгБҝөДNaOHИЬТә»мәПјУИИЈә
NH4Ј«Ј«HSO3ЈӯЈ«2OHЈӯ NH3?Ј«SO32ЈӯЈ«2H2O NH3?Ј«SO32ЈӯЈ«2H2O
CЈ®ЛбРФМхјюПВKIO3ИЬТәУлKIИЬТә·ўЙъ·ҙУҰЙъіЙI2Јә
IO3ЈӯЈ«5IЈӯЈ«3H2OЈҪ3I2Ј«6OHЈӯ
DЈ®AgNO3ИЬТәЦРјУИл№эБҝөД°ұЛ®ЈәAgЈ«Ј«NH3ЎӨH2OЈҪAgOHЈ«NH4Ј«
ІОҝјҙр°ёЈәBЎЈ
ұҫМвҪвОцЈәСЎПоAЦРCa(OH)2ОӘОўИЬРФОпЦКЈ¬ЛщТФКҜ»ТИйөД»ҜС§КҪІ»ДЬёДРҙЈ»СЎПоCЦРФЪЛбРФИЬТәЦРOHЈӯУлHЈ«І»ДЬ№ІҙжЈ»СЎПоDЦРөДAgOHТӘҪшТ»ІҪУлNH3ЎӨH2O·ҙУҰЎЈ
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ПВБРҪвКНКВКөөД·ҪіМКҪІ»ХэИ·өДКЗЈЁ?Ј©
AЈ®Ів0.1mol/L°ұЛ®өДpHОӘ11ЈәNH3ЎӨH2O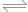 NH4++OH- NH4++OH-
BЈ®Ҫ«Naҝй·ЕИлЛ®ЦРЈ¬ІъЙъЖшМеЈә2Na+2H2O=2NaOH+H2Ўь
CЈ®УГCuCl2ИЬТәЧцөјөзКөСйЈ¬өЖЕЭ·ў№вЈәCuCl2 Cu2++2Cl- Cu2++2Cl-
DЈ®AlЖ¬ИЬУЪNaOHИЬТәЦРЈ¬ІъЙъЖшМеЈә2Al+2OH-+2H2O=2AlO2-+3H2Ўь
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈәAЎў°ұЛ®ОӘИхөзҪвЦКЈ¬ҙжФЪөзАлЖҪәвЈ¬ХэИ·Ј»BЎўNaРФЦК·ЗіЈ»оЖГЈ¬ҝЙУлЛ®·ҙУҰ·ЕіцH2Ј¬ХэИ·Ј»CЎўөзАлұҫЙнІ»РиТӘНЁөзЈ¬№К·ҪіМКҪҙнОуЈ»DЎўAlҝЙУлNaOHИЬТә·ҙУҰ·ЕіцЗвЖшЈ¬ХэИ·ЎЈ
ұҫМвДС¶ИЈәТ»°г
|