1ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪЈ¬КйРҙХэИ·өДКЗ?ЈЁ?Ј©
AЈ®МъәНПЎСОЛб·ҙУҰ 2Fe+6H+===2Fe3++3H2Ўь
BЈ®ПхЛбТшИЬТәЦРјУИлНӯ·Ы Ag++Cu===Cu2++Ag
CЈ®Сх»ҜНӯУлСОЛб·ҙУҰ O2ЁD +2HЈ« ===H2O
DЈ®МјЛбГҫёъПЎБтЛб·ҙУҰЈәMgCO3Ј«2HЈ«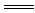 Mg2Ј«Ј«H2OЈ«CO2Ўь
Mg2Ј«Ј«H2OЈ«CO2Ўь
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәAҙнЈ¬ХэИ·ОӘFe+2H+===Fe2++H2ЎьЈ»BҙнЈ¬өзәЙОӘКШәгЈ¬ХэИ·ОӘ2Ag++Cu===Cu2++2AgЈ»CҙнЈ¬ХэИ·ОӘЈәCuO +2HЈ« ===Cu2++H2OЈ»DХэИ·Ј»
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®Cl2УлNaOHИЬТә·ҙУҰЈәCl2+OH-ЁTCl-+ClO-+H2O
BЈ®ЗвСх»ҜГҫУлБтЛбИЬТә·ҙУҰOH-+H+=H2O
CЈ®МјЛбёЖУлҙЧЛбИЬТә·ҙУҰЈәCaCO3+2H+=Ca2++H2O+CO2Ўь
DЈ®іОЗеКҜ»ТЛ®ЦРјУИлМјЛбДЖИЬТәЈәCa2++CO32-ЁTCaCO3Ўэ
ІОҝјҙр°ёЈәAЈ®Cl2УлNaOHИЬТә·ҙУҰөДАлЧУ·ҙУҰОӘCl2+2OH-ЁTCl-+ClO-+H2OЈ¬№КAҙнОуЈ»
BЈ®ЗвСх»ҜГҫУлБтЛбИЬТә·ҙУҰөДАлЧУ·ҙУҰОӘMgЈЁOHЈ©2+H+=H2O+Mg2+Ј¬№КBҙнОуЈ»
CЈ®МјЛбёЖУлҙЧЛбИЬТә·ҙУҰөДАлЧУ·ҙУҰОӘCaCO3+2HAc=Ca2++H2O+CO2Ўь+2Ac-Ј¬№КCҙнОуЈ»
DЈ®КҜ»ТЛ®ЦРјУИлМјЛбДЖИЬТә·ҙУҰөДАлЧУ·ҙУҰОӘCa2++CO32-ЁTCaCO3ЎэЈ¬№КDХэИ·Ј»
№КСЎDЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ДЬУГH++OH-=H2OұнКҫөД·ҙУҰОӘЈЁЎЎЎЎЈ©
AЈ®ЗвСх»ҜјШИЬТәУлБтЛб·ҙУҰ
BЈ®ЗвСх»ҜұөИЬТәУлБтЛб·ҙУҰ
CЈ®ЗвСх»ҜНӯУлПЎПхЛб·ҙУҰ
DЈ®ЗвСх»ҜДЖИЬТәУлҙЧЛб·ҙУҰ
ІОҝјҙр°ёЈәAЎўБтЛбОӘЗҝЛбЎўЗвСх»ҜјШОӘЗҝјоЈ¬ЙъіЙҝЙИЬРФСОәНЛ®Ј¬ДЬУГАлЧУ·ҪіМКҪH++OHЁTH2OұнКҫЈ¬№КAХэИ·Ј»
BЎўЗвСх»ҜұөУлПЎБтЛб·ҙУҰЈ¬·ҙУҰІъОп»№УРБтЛбұөіБөнЙъіЙЈ¬І»ДЬУГH++OH-=H2OұнКҫЈ¬№КBҙнОуЈ»
CЎўЗвСх»ҜНӯОӘИхјоЈ¬ФЪАлЧУ·ҙУҰЦРРҙіЙ»ҜС§КҪЈ¬І»ДЬУГАлЧУ·ҪіМКҪH++OHЁTH2OұнКҫЈ¬№КCҙнОуЈ»
DЎўҙЧЛбКфУЪИхөзҪвЦКЈ¬АлЧУ·ҪіМКҪЦРРҙіЙ·ЦЧУКҪЈ¬І»ДЬУГАлЧУ·ҪіМКҪH++OHЁTH2OұнКҫЈ¬№КDҙнОуЈ»
№КСЎЈәAЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
4ЎўМоҝХМв (1)Ҫ«Лйө°ҝЗЈЁЦчТӘіЙ·ЦКЗCaCO3Ј©ҪюЕЭФЪСОЛбЦР»бЙъіЙТ»ЦЦДЬК№іОЗеКҜ»ТЛ®ұд»лЧЗөДОЮЙ«ЖшМеЎЈЙПКцөДБҪёц·ҙУҰөДАлЧУ·ҪіМКҪОӘЈәўЩ?
?ўЪ?
(2)ОТГЗіЈФЪИИЛ®ЖҝЦРҝҙөҪЛ®№ёЈ¬ЖдЦчТӘіЙ·ЦКЗCaCO3әНMg(OH)2Ј¬ТӘіэИҘ Л®№ёЈ¬іЈУГөД·Ҫ·ЁКЗПтЖҝЦРјУИлПЎҙЧЛбҪ«ЖдИЬҪвЈ¬Жд·ҙУҰөДАлЧУ·ҪіМКҪОӘўЩ?
Л®№ёЈ¬іЈУГөД·Ҫ·ЁКЗПтЖҝЦРјУИлПЎҙЧЛбҪ«ЖдИЬҪвЈ¬Жд·ҙУҰөДАлЧУ·ҪіМКҪОӘўЩ?
?ўЪ?
(3)MgSO4ИЬТәЦРөОјУЙЩБҝ°ұЛ®Ј¬Жд·ҙУҰөДАлЧУ·ҪіМКҪОӘ?
(4)ЗлҪ«ПВБРАлЧУ·ҪіМКҪёДРҙОӘ»ҜС§·ҪіМКҪЈә
ўЩ 2NH4+ + Ba2+ + 2OH- + SO42- = BaSO4Ўэ+2NH3ЎӨH2O
?
ўЪ BaCO3 + 2H+ = Ba2++CO2Ўь+H2O
?
ІОҝјҙр°ёЈәўЕўЩ CaCO3 + 2H+ = Ca2+ + CO2Ўь+H2O
ўЪ CO2 + Ca2+ + 2OHЎӘ = CaCO3Ўэ+ H2O
ўЖўЩ CaCO3 + 2CH3COOH = Ca2+ + 2CH3COOЎӘ?+CO2Ўь+H2O
ўЪ Mg(OH)2 + 2CH3COOH = Mg2+ + 2CH3COOЎӘ?+2 H2O
ўЗ Mg2++2 NH3ЎӨH2O =Mg(OH)2Ўэ+2NH4+
ўИ ўЩ (NH4)2SO4 + Ba(OH)2 = BaSO4Ўэ+2 NH3ЎӨH2O
ўЪ BaCO3 + 2HCl = BaCl2 + H2O + CO2ЎьЈЁЖдЛыәПАнҙр°ёТІҝЙЈ©ЈЁёч2·ЦЈ©
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ
5ЎўСЎФсМв ПВБРҪбәПКөСйПЦПуөД·ҙУҰ·ҪіМКҪІ»ХэИ·өДКЗ
AЈ®ФЪ·ЕУРҪрКфДЖөДјҜЖшЖҝЦРНЁИлВИЖшЈ¬ІъЙъ°ЧСМ 2Na+Cl2=2NaCl
BЈ®ПтNaHCO3ИЬТәЦРјУИл№эБҝөДіОЗеКҜ»ТЛ®Ј¬іцПЦ°ЧЙ«іБөн
2HCO3-+Ca2++2OH-=CaCO3Ўэ+CO32-+2H2O
CЈ®ФЪ0.1 molЎӨL-1өДK2Cr2O7ИЬТәЦРөОјУКэөО1molЎӨL-1өДNaOHИЬТәЈ¬ИЬТәУЙіИЙ«ұдіЙ»ЖЙ«
Cr2O72-+H2O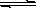 2CrO42-+2H+
2CrO42-+2H+
DЈ®С№ЛхЧ°УРNO2ЖшМеөДХлНІЈ¬әмЧШЙ«ПИұдЙоәуұдЗіө«ұИФӯАҙ»№КЗТӘЙо2NO2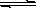 N2O4
N2O4
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәДЖУлВИЖшЙъіЙВИ»ҜДЖ№ММеЈ¬ІъЙъ°ЧСМЈ¬AХэИ·Ј»NaHCO3ИЬТәЦРјУИл№эБҝөДіОЗеКҜ»ТЛ®Ј¬Ca2+әНOH-ёцКэұИІ»КЗ1Јә2Ј¬·ҙУҰАлЧУ·ҪіМКҪОӘHCO3-+Ca2++OH-=CaCO3Ўэ+H2OЈ¬BҙнОуЈ»ПтЦШёхЛбјШИЬТәЦРСх»ҜДЖИЬТәЈ¬OH-УлH+·ҙУҰЈ¬ЖҪәвПтУТТЖ¶ҜЈ¬ИЬТәұд»ЖЈ¬CХэИ·Ј»С№ЛхЧ°УР¶юСх»ҜөӘЖшМеөДХлНІЈ¬ЖшМеЕЁ¶ИФцҙуЈ¬СХЙ«јУЙоЈ¬И»әуЖҪәвПтУТТЖ¶ҜЈ¬СХЙ«ұдЗіЈ¬ө«ұИФӯАҙЙоЈ¬DХэИ·ЎЈ
өгЖАЈәҪ«»ҜС§»щұҫАнВЫУл»ҜС§·ҪіМКҪЧЫәПҝјІйЈ¬ЛјО¬ИЭБҝҙуЎЈ
ұҫМвДС¶ИЈәТ»°г