1ЎўСЎФсМв ДЬХэИ·ұнКҫПВБР»ҜС§·ҙУҰөДАлЧУ·ҪіМКҪКЗ
AЈ®FeOИЬУЪ№эБҝПЎПхЛбЈә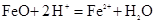
BЈ®ВИ»Ҝп§әНКмКҜ»ТЦЖ°ұЖшЈә
CЈ®ВИ»ҜВБәНЛДфЗ»щәПВБЛбДЖИЬТә»мәПЈә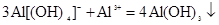
DЈ®МјЛбёЖИЬУЪҙЧЛбЦРЈә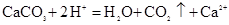
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ХэИ·ҙр°ёЈәC
AЎўІ»ХэИ·Ј¬3FeOЈ«10HЈ«Ј«NO3ЁD=3Fe3Ј«Ј«NOЈ«5H2O
BЎў№ММеЈ¬І»РҙАлЧУ·ҪіМ
CЎўХэИ·Ј»
DЎўІ»ХэИ·Ј¬CaCO3Ј«2CH3COOH=Ca2Ј«Ј«2CH3COOЁDЈ«H2OЈ«CO2Ўь
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗ?
ўЩЈ®өИМе»эөИОпЦКөДБҝЕЁ¶ИөДЗвСх»ҜұөПЎИЬТәУлМјЛбЗвп§ПЎИЬТә»мәП
Ba2+ + 2OH ЁC + NH4+ + HCO3 ЁC = BaCO3Ўэ+ NH3ЎӨH2O + H2O
ўЪЈ®ВИ»ҜВБИЬТәЦРјУИл№эБҝөД°ұЛ®? Al3+ + 4NH3ЎӨH2O = 4NH4+ + AlO2 ЁC + 2H2O
ўЫЈ®ПтҙОВИЛбёЖИЬТәЦРНЁИл№эБҝөД¶юСх»ҜБт? Ca2+ + 2ClO ЁC + SO2 + H2O = CaSO3Ўэ+ 2HClO
ўЬЈ®ТСЦӘөИЕЁ¶ИөДМјЛбДЖЎўҙОВИЛбДЖЎўМјЛбЗвДЖPHЦрҪҘјхРЎЈ¬ПтҙОВИЛбДЖИЬТәНЁЙЩБҝ¶юСх»ҜМј
ClO- +CO2 +H2O? =" HClO" + CO32-
ўЭЈ®№эСх»ҜДЖ№ММеУлЛ®·ҙУҰЈә2O22?+2H2OЈҪ4OH?+O2Ўь
ўЮЈ®°ұЛ®ЦРНЁИл№эБҝ¶юСх»ҜБтЈә2NH3?H2O + SO2 = 2NH4+ +SO32? +H2O
ўЯЈ®іОЗеөДКҜ»ТЛ®ЦРјУИЛ№эБҝөДNaHCO3ИЬТәЈәCa2++OH?+HCO3?=CaCO3Ўэ+H2O
ўаЈ®Ҫ«2mol/LAlCl3ИЬТәәН7mol/LNaOHИЬТәөИМе»э»мәПЈә
2Al3Ј«Ј«7OH?ЈҪAl(OH)3ЎэЈ«AlO2?Ј«2H2O?
ўбЎўПтBa(OH)2ИЬТәЦРөОјУNaHSO4ИЬТәЦБЗЎәГОӘЦРРФЈәBa2+ + 2OH- + 2H+ + SO42-?= BaSO4Ўэ+ 2H2O
AЈ®ўЩўЫўЮўб
BЈ®ўЪўЬўЭўб
CЈ®ўЫўЬўЯўа
DЈ®ўЩўаўб
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәўЩ·ыәПҝН№ЫКВКөЈ¬ІрРҙ·ыәПФӯФтЎЈХэИ·ЎЈўЪЈ®Т»Л®әП°ұКЗИхјоЈ¬І»ДЬИЬҪвЗвСх»ҜВБЎЈ·ҙУҰөДАлЧУ·ҪіМКҪКЗЈәAl3+ + 3NH3ЎӨH2O = 3NH4+ + Al(OH)3 Ўэ.ҙнОуЎЈўЫHClOУРЗҝСх»ҜРФЈ¬»б·ўЙъСх»Ҝ»№Фӯ·ҙУҰЈ¬АлЧУ·ҪіМКҪКЗCa2+ + ClO ЁC + SO2 + H2O = CaSO4Ўэ+ 2H++ClЈӯЎЈҙнОуЎЈўЬЛбРФЈәH2CO3>HClO> HCO3?Ј¬Ҫб№№ёҙ·ЦҪв·ҙУҰөД№жВЙКЗЗҝЛбЦЖИЎИхЛбЈ¬ЛщТФ·ўЙъ·ҙУҰЈәClO- +CO2 +H2O? =" HClO" + HCO3-ЎЈҙнОуЎЈўЭ№эСх»ҜДЖ№ММеРҙ»ҜС§КҪЈ¬·ҪіМКҪКЗЈә2Na2O22?+2H2OЈҪ4Na++4OH?+O2Ўь.ҙнОуЎЈўЮ¶юСх»ҜБт№эБҝЈ¬өГөҪЛбКҪСОЎЈ·ҪіМКҪКЗЈә NH3?H2O + SO2 = NH4+ +HSO3? ЎЈҙнОуЎЈўЯјоУлЛбКҪСО·ҙУҰКұТӘТФІ»ЧгБҝөДОпЦКОӘұкЧјЎЈАлЧУ·ҪіМКҪКЗCa2++2OH?+2HCO3?=CaCO3Ўэ+2H2O+CO32-ЎЈҙнОуЎЈўаўб·ыәПКВКөЎЈХэИ·ЎЈТтҙЛХэИ·өДЛө·ЁКЗўЩўаўбЎЈСЎПоОӘDЎЈ
ұҫМвДС¶ИЈәТ»°г
3ЎўМоҝХМв ПтГч·Ҝ[KAl(SO4)2ЎӨ12H2O]ИЬТәЦРЦрөОјУИлBa(OH)2ИЬТәЦБ№эБҝЎЈ
(1)РҙіцҝЙДЬ·ўЙъөДУР№Ш·ҙУҰөД»ҜС§·ҪіМКҪЎЈ?ЎЈ
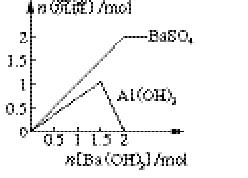
(2)ФЪНјЦРЈ¬ЧЭЧшұкұнКҫЙъіЙіБөнөДОпЦКөДБҝЈ¬әбЧшұкұнКҫјУИлBa(OH)2өДОпЦКөДБҝЎЈКФФЪНјЦР»ӯіцөұИЬТәЦРГч·ҜОӘ1? molКұЈ¬ЙъіЙөДГҝТ»ЦЦіБөнөДОпЦКөДБҝЛжBa(OH)2ОпЦКөДБҝұд»ҜөДЗъПЯ(ФЪЗъПЯЙПұкГчіБөнөД»ҜС§КҪ)ЎЈ
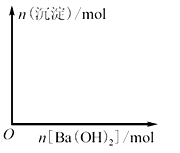
(3)Птa? LОпЦКөДБҝЕЁ¶ИОӘM? mol/LөДГч·ҜИЬТәЦРөОјУОпЦКөДБҝЕЁ¶ИОӘn? mol/LөДBa(OH)2ИЬТәb? LЈ¬УГә¬aЎўMЎўnөДұнҙпКҪұнКҫЈә
ўЩөұbВъЧг________МхјюКұЈ¬іБөнөДЧЬОпЦКөДБҝОӘЧоҙуЦөЈ»
ўЪөұbВъЧг________МхјюКұЈ¬іБөнөДЦКБҝОӘЧоҙуЦөЎЈ
ІОҝјҙр°ёЈә(1)2KAl(SO4)2Ј«3Ba(OH)2ЈҪK2SO4Ј«3BaSO4ЎэЈ«2Al(OH)3ЎэЈЁ1·ЦЈ©
K2SO4Ј«2Al(OH)3Ј«Ba(OH)2ЈҪ2KAlO2Ј«BaSO4ЎэЈ«4H2OЈЁ1·ЦЈ©
(2)? ЈЁ4·ЦЈ©(3)ўЩ1.5aMЈҪnbЈЁ1·ЦЈ©?ўЪ2aMЈҪnbЈЁ1·ЦЈ©
ЈЁ4·ЦЈ©(3)ўЩ1.5aMЈҪnbЈЁ1·ЦЈ©?ўЪ2aMЈҪnbЈЁ1·ЦЈ©
ұҫМвҪвОцЈәЈЁ1Ј©өұГч·ҜЦРВБАлЧУЗЎәГРОіЙЗвСх»ҜВБіБөнКұЈ¬·ҙУҰөД»ҜС§·ҪіМКҪОӘ2KAl(SO4)2Ј«3Ba(OH)2ЈҪK2SO4Ј«3BaSO4ЎэЈ«2Al(OH)3ЎэЈ»УЙУЪЗвСх»ҜВБКЗБҪРФЗвСх»ҜОпЈ¬ЛщТФөұГч·ҜЦРSO42ЈӯЗЎәГ·ҙУҰЙъіЙБтЛбұө°ЧЙ«іБөнКұЈ¬ҙЛКұИЬТәЦР·ҙУҰөДАлЧУ·ҪіМКҪОӘK2SO4Ј«2Al(OH)3Ј«Ba(OH)2ЈҪ2KAlO2Ј«BaSO4ЎэЈ«4H2OЎЈ
ЈЁ2Ј©ёщҫЭ·ҙУҰКҪ2KAl(SO4)2Ј«3Ba(OH)2ЈҪK2SO4Ј«3BaSO4ЎэЈ«2Al(OH)3ЎэҝЙЦӘЈ¬өұјУИл1.5molЗвСх»ҜұөКұЙъіЙ1.5molБтЛбұөәН1molЗвСх»ҜВБЎЈёщҫЭ·ҙУҰКҪKAl(SO4)2Ј«2Ba(OH)2ЈҪKAlO2Ј«2BaSO4ЎэЈ«2H2OҝЙЦӘЈ¬өұјУИл2molЗвСх»ҜұөКұЈ¬ЙъіЙөДЗвСх»ҜВБУЦИЬҪвЙъіЙЖ«ВБЛбДЖЈ¬ҙЛКұБтЛбұөөДіБөнҙпөҪЧоҙуЦөЈ¬ТтҙЛНјПсҝЙұнКҫОӘ ЎЈ
ЎЈ
ЈЁ3Ј©ўЩёщҫЭ·ҙУҰ2KAl(SO4)2Ј«3Ba(OH)2ЈҪK2SO4Ј«3BaSO4ЎэЈ«2Al(OH)3ЎэәНKAl(SO4)2Ј«2Ba(OH)2ЈҪKAlO2Ј«2BaSO4ЎэЈ«2H2OҝЙЦӘЈ¬өұіБөнөДОпЦКөДБҝЧоҙуКұГч·ҜУлЗвСх»ҜұөөДОпЦКөДБҝЦ®ұИЈҪ2:3Ј¬Фт1.5aMЈҪnbЎЈ
ўЪН¬СщёщҫЭ·ҙУҰҝЙЦӘЈ¬өұЗЎәГЙъіЙБтЛбұөіБөнКұіБөнЦКБҝЧоҙуЈ¬ҙЛКұГч·ҜУлЗвСх»ҜұөөДОпЦКөДБҝЦ®ұИЈҪ1:2Ј¬Фт2aMЈҪnbЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ПВБРёчАлЧУ·ҪіМКҪХэИ·өДКЗЈЁЎЎЎЎЎЎЎЎЎЎЈ©
ЈБМъЖ¬Н¶ИлПЎБтЛбЦРЈә2Fe + 6H ="===" 2Fe
="===" 2Fe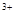 + 3H2Ўь
+ 3H2Ўь
ЈВУГКҜ»ТКҜУлСОЛб·ҙУҰЦЖИЎCOЈәCO32-+ 2H+="===" H2O + CO2Ўь
ЈГ Ba (OH)2ИЬТәЦРјУИлПЎБтЛбЈәBa2++ SO42-="===" BaSO4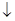 ?
?
ЈДҪрКфДЖУлЛ®·ҙУҰЈәЈІNa +ЈІ H2O ="===" ЈІNa + 2OH
+ 2OH + H2Ўь
+ H2Ўь
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәAҙнЈ¬ХэИ·ОӘЈәFe + 2H ="===" Fe2++ H2ЎьЈ»BҙнЈ¬ХэИ·ОӘЈәКҜ»ТКҜОӘДСИЬУЪЛ®өДОпЦКЈ¬І»ДЬІріЙАлЧУРОКҪЈ»CҙнЈ¬іэБЛЙъіЙБтЛбұөНв»№УРЛ®ЙъіЙЈ»DХэИ·Ј»
="===" Fe2++ H2ЎьЈ»BҙнЈ¬ХэИ·ОӘЈәКҜ»ТКҜОӘДСИЬУЪЛ®өДОпЦКЈ¬І»ДЬІріЙАлЧУРОКҪЈ»CҙнЈ¬іэБЛЙъіЙБтЛбұөНв»№УРЛ®ЙъіЙЈ»DХэИ·Ј»
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв УлАлЧУ·ҪіМКҪFe3++3OH-=Fe(OH)3ЎэПа¶ФУҰөД»ҜС§·ҪіМКҪКЗ
[? ]
A. FeSO4+2NaOH=Fe(OH)2Ўэ+Na2SO4
B. 2Fe(NO3)3+3Mg(OH)2=2Fe(OH)3Ўэ+3Mg(NO3)2
C. Fe2(SO4)3+3Ba(OH)2=2Fe(OH)3Ўэ+3BaSO4Ўэ
D. Fe2(SO4)3+6NaOH=2Fe(OH)3Ўэ+3Na2SO4
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ