1ЎўСЎФсМв ПВБР»ҜС§·ҪіМКҪДЬУГАлЧУ·ҪіМКҪCO32-+2H+ЁTH2O+CO2ЎьұнКҫөДКЗЈЁЎЎЎЎЈ©
AЈ®CaCO3+2HClЁTCaCl2+CO2Ўь+H2O
BЈ®NaHCO3+CH3COOHЁTCH3COONa+CO2Ўь+H2O
CЈ®Na2CO3+2CH3COOHЁT2CH3COONa+CO2Ўь+H2O
DЈ®Na2CO3+H2SO4ЁTNa2SO4+CO2Ўь+H2O
2ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪКйРҙҙнОуөДКЗЈЁЎЎЎЎЈ©
AЈ®CH3COOHИЬТәУлNaOHИЬТә·ҙУҰ CH3COOH+OH-=CH3COO-+H2O
BЈ®ПЎСОЛбөОИл°ұЛ®ЦРH++OH-=H2O
CЈ®Сх»ҜВБ№ММеУлЙХјоИЬТә»мәПAl2O3+2OH-=2AlO2-+H2O
DЈ®ПЎБтЛбөОФЪНӯЖ¬ЙПCu+2H+=Cu2++H2Ўь
3ЎўСЎФсМв ФЪБтЛбНӯИЬТәЦРјУИлөв»ҜјШИЬТәЈ¬УР°ЧЙ«іБөнЙъіЙЈ¬ИЬТәөДСХЙ«ФтУЙА¶Й«ұдОӘЙо»ЖЙ«Ј¬ҫӯ·ЦОцЦӨГч°ЧЙ«іБөнКЗөв»ҜСЗНӯЎЈұнКҫХвёцСх»Ҝ»№Фӯ·ҙУҰөДАлЧУ·ҪіМКҪКЗ
[? ]
A.?Cu2+Ј«3IЈӯ===CuIЎэЈ«I2?
B.?Cu2+Ј«2IЈӯ===Cu+Ј«I2
C.?2Cu2+Ј«2IЈӯ===2Cu+Ј«I2?
D.?2Cu2+Ј«4IЈӯ===2CuIЎэЈ«I2
4ЎўјтҙрМв °ҙТӘЗуНкіЙПВБРМоҝХ
ЈЁ1Ј©РҙіцПВБРОпЦКөДөзАл·ҪіМКҪЈәFe2ЈЁSO4Ј©3______Ј¬NaHCO3______Ј»
ЈЁ2Ј©РҙіцПВБР·ҙУҰөДАлЧУ·ҪіМКҪЈәПЎСОЛбУлМјЛбёЖ·ҙУҰ______Ј¬ЗвСх»ҜұөИЬТәУлПЎБтЛб·ҙУҰ______Ј»
ЈЁ3Ј©РҙіцУлПВБРАлЧУ·ҪіМКҪПа¶ФУҰөД»ҜС§·ҪіМКҪЈәH++OH-ЁTH2O______Ј¬CO32-+2H+ЁTCO2Ўь+H2O______Ј®
5ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®јЧИ©әНЧгБҝТш°ұИЬТәід·Ц·ҙУҰЈә
HCHO+2AgЈЁNH3Ј©2++2OH-
HCOO-+NH4++2AgЎэ+H2O+3NH3
BЈ®Птә¬УРБтЛбёЖөДЛ®№ёЦРјУИлМјЛбДЖИЬТәЈ¬іБөнөДЧӘ»ҜҝЙұнКҫОӘЈә
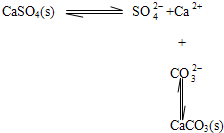
CЈ®ЛбРФМхјюПВKIO3ИЬТәУлKIИЬТә·ўЙъ·ҙУҰЙъіЙI2ЈәIO3-+5I-+3H2O=3I2+6OH
DЈ®деТТНйЦРөОИлAgNO3ИЬТәјмСйЖдЦРөДдеФӘЛШЈәBr-+Ag+=AgBrЎэ