1ЎўСЎФсМв ПВБР»ҜС§·ҪіМКҪДЬУГАлЧУ·ҪіМКҪCO32-+2H+ЁTH2O+CO2ЎьұнКҫөДКЗЈЁЎЎЎЎЈ©
AЈ®CaCO3+2HClЁTCaCl2+CO2Ўь+H2O
BЈ®NaHCO3+CH3COOHЁTCH3COONa+CO2Ўь+H2O
CЈ®Na2CO3+2CH3COOHЁT2CH3COONa+CO2Ўь+H2O
DЈ®Na2CO3+H2SO4ЁTNa2SO4+CO2Ўь+H2O
ІОҝјҙр°ёЈәAЈ®CaCO3ОӘДСИЬОпЈ¬УҰёГРҙ»ҜС§КҪЈ¬№КAҙнОуЈ»
BЈ®МјЛбЗвДЖөзАліцКЗHCO3-¶шІ»КЗCO32-Ј¬ЗТҙЧЛбКЗИхөзҪвЦКТӘРҙ»ҜС§КҪЈ¬№КBҙнОуЈ»
CЈ®ҙЧЛбКЗИхөзҪвЦКТӘРҙ»ҜС§КҪЈ¬№КCҙнОуЈ»
DЈ®Na2CO3ЎўH2SO4ЎўNa2SO4¶јКЗҝЙИЬРФЗҝөзҪвЦКЈ¬ТӘРҙАлЧУЈ¬ЛщТФЖдАлЧУ·ҪіМКҪОӘCO32-+2H+ЁTH2O+CO2ЎьЈ¬№КDХэИ·Ј»
№КСЎDЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
2ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪКйРҙҙнОуөДКЗЈЁЎЎЎЎЈ©
AЈ®CH3COOHИЬТәУлNaOHИЬТә·ҙУҰ CH3COOH+OH-=CH3COO-+H2O
BЈ®ПЎСОЛбөОИл°ұЛ®ЦРH++OH-=H2O
CЈ®Сх»ҜВБ№ММеУлЙХјоИЬТә»мәПAl2O3+2OH-=2AlO2-+H2O
DЈ®ПЎБтЛбөОФЪНӯЖ¬ЙПCu+2H+=Cu2++H2Ўь
ІОҝјҙр°ёЈәAЈ®CH3COOHИЬТәУлNaOHИЬТә·ҙУҰөДАлЧУ·ҙУҰОӘCH3COOH+OH-=CH3COO-+H2OЈ¬№КAХэИ·Ј»
BЈ®ПЎСОЛбөОИл°ұЛ®ЦРөДАлЧУ·ҙУҰОӘH++NH3Ј®H2O=NH4++H2OЈ¬№КBҙнОуЈ»
CЈ®Сх»ҜВБ№ММеУлЙХјоИЬТә»мәПөДАлЧУ·ҙУҰОӘAl2O3+2OH-=2AlO2-+H2OЈ¬№КCХэИ·Ј»
DЈ®CuУлПЎБтЛбІ»·ҙУҰЈ¬І»ДЬКйРҙАлЧУ·ҙУҰЈ¬№КDҙнОуЈ»
№КСЎBDЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ФЪБтЛбНӯИЬТәЦРјУИлөв»ҜјШИЬТәЈ¬УР°ЧЙ«іБөнЙъіЙЈ¬ИЬТәөДСХЙ«ФтУЙА¶Й«ұдОӘЙо»ЖЙ«Ј¬ҫӯ·ЦОцЦӨГч°ЧЙ«іБөнКЗөв»ҜСЗНӯЎЈұнКҫХвёцСх»Ҝ»№Фӯ·ҙУҰөДАлЧУ·ҪіМКҪКЗ
[? ]
A.?Cu2+Ј«3IЈӯ===CuIЎэЈ«I2?
B.?Cu2+Ј«2IЈӯ===Cu+Ј«I2
C.?2Cu2+Ј«2IЈӯ===2Cu+Ј«I2?
D.?2Cu2+Ј«4IЈӯ===2CuIЎэЈ«I2
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
4ЎўјтҙрМв °ҙТӘЗуНкіЙПВБРМоҝХ
ЈЁ1Ј©РҙіцПВБРОпЦКөДөзАл·ҪіМКҪЈәFe2ЈЁSO4Ј©3______Ј¬NaHCO3______Ј»
ЈЁ2Ј©РҙіцПВБР·ҙУҰөДАлЧУ·ҪіМКҪЈәПЎСОЛбУлМјЛбёЖ·ҙУҰ______Ј¬ЗвСх»ҜұөИЬТәУлПЎБтЛб·ҙУҰ______Ј»
ЈЁ3Ј©РҙіцУлПВБРАлЧУ·ҪіМКҪПа¶ФУҰөД»ҜС§·ҪіМКҪЈәH++OH-ЁTH2O______Ј¬CO32-+2H+ЁTCO2Ўь+H2O______Ј®
ІОҝјҙр°ёЈәЈЁ1Ј©Fe2ЈЁSO4Ј©3өДFe2ЈЁSO4Ј©3ЁT2Fe3++3SO42-Ј¬NaHCO3өДөзАл·ҪіМКҪОӘЈәNaHCO3ЁTNa++HCO3-Ј¬№Кҙр°ёОӘЈәFe2ЈЁSO4Ј©3ЁT2Fe3++3SO42-Ј»NaHCO3ЁTNa++HCO3-Ј»
ЈЁ2Ј©МјЛбСОҝЙТФәНСОЛб·ҙУҰЈ¬КөЦККЗәНЛбЦРЗвАлЧУөД·ҙУҰЈ¬ПЎСОЛбУлМјЛбёЖ·ҙУҰөДАлЧУ·ҪіМКҪОӘЈәCaCO3+2H+ЁTCa2++CO2Ўь+H2OЈ¬ЗвСх»ҜұөҝЙТФәНБтЛб·ўЙъЦРәН·ҙУҰЙъіЙБтЛбұөәНЛ®Ј¬КөЦККЗЈәBa2++2OH-+2H++SO42-ЁTBaSO4Ўэ+2H2OЈ¬№Кҙр°ёОӘЈәCaCO3+2H+ЁTCa2++CO2Ўь+H2OЈ»Ba2++2OH-+2H++SO42-ЁTBaSO4Ўэ+2H2OЈ»
ЈЁ3Ј©H++OH-ЁTH2OұнКҫҝЙИЬРФөДЗҝЛбәНҝЙИЬРФөДЗҝјо·ҙУҰЙъіЙҝЙИЬРФөДСОТФј°ЛҜөГТ»Аа»ҜС§·ҙУҰЈ¬ИзЈәHCl+NaOHЁTNaCl+H2OЈ»CO32-+2H+ЁTCO2Ўь+H2OұнКҫҝЙИЬРФөДМјЛбСОәНЗҝЛб·ҙУҰЙъіЙҝЙИЬРФөДСОәНЛ®ТФј°¶юСх»ҜМјөД·ҙУҰЈ¬ИзЈәNa2CO3+2HClЁT2NaCl+CO2Ўь+H2OЈ¬№Кҙр°ёОӘЈәHCl+NaOHЁTNaCl+H2OЈ»Na2CO3+2HClЁT2NaCl+CO2Ўь+H2OЈЁЖдЛыәПАнҙр°ёТІҝЙөГ·ЦЈ©Ј®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®јЧИ©әНЧгБҝТш°ұИЬТәід·Ц·ҙУҰЈә
HCHO+2AgЈЁNH3Ј©2++2OH-
HCOO-+NH4++2AgЎэ+H2O+3NH3
BЈ®Птә¬УРБтЛбёЖөДЛ®№ёЦРјУИлМјЛбДЖИЬТәЈ¬іБөнөДЧӘ»ҜҝЙұнКҫОӘЈә
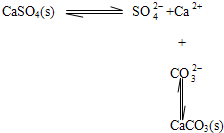
CЈ®ЛбРФМхјюПВKIO3ИЬТәУлKIИЬТә·ўЙъ·ҙУҰЙъіЙI2ЈәIO3-+5I-+3H2O=3I2+6OH
DЈ®деТТНйЦРөОИлAgNO3ИЬТәјмСйЖдЦРөДдеФӘЛШЈәBr-+Ag+=AgBrЎэ
ІОҝјҙр°ёЈәAЈ®јЧИ©әНЧгБҝТш°ұИЬТәід·Ц·ҙУҰөДАлЧУ·ҙУҰОӘHCHO+4AgЈЁNH3Ј©2++4OH-?Ўч?.?CO32-+2NH4++4AgЎэ+2H2O+6NH3Ј¬№КAҙнОуЈ»
BЈ®Птә¬УРБтЛбёЖөДЛ®№ёЦРјУИлМјЛбДЖИЬТәЈ¬МјЛбёЖөДИЬҪв¶ИёьРЎЈ¬ФтБтЛбёЖЧӘ»ҜОӘМјЛбёЖіБөнЈ¬№КBХэИ·Ј»
CЈ®ЛбРФМхјюПВKIO3ИЬТәУлKIИЬТә·ўЙъ·ҙУҰЙъіЙI2өДАлЧУ·ҙУҰОӘIO3-+5I-+6H+=3I2+3H2OЈ¬№КCҙнОуЈ»
DЈ®деТТНйЦРІ»ҙжФЪдеАлЧУЈ¬ФтдеТТНйЦРөОИлAgNO3ИЬТәІ»·ҙУҰЈ¬№КDҙнОуЈ»
№КСЎBЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ