1ЎўјтҙрМв №ӨТө·ПЛ®ЦРіЈә¬УРТ»¶ЁБҝөДCr2O72-әНCrO42-Ј¬ЛьГЗ»б¶ФИЛАај°ЙъМ¬ПөНіІъЙъәЬҙуөДЙЛәҰЈ¬ұШРлҪшРРҙҰАнЈ®Ді№Өі§К№УГ»№ФӯіБөн·ЁҙҰАнЈ¬ёГ·ЁөД№ӨТХБчіМОӘЈә
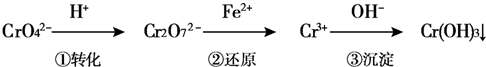
ЖдЦРөЪўЩІҪҙжФЪЖҪәвЈә2CrO42-ЈЁ»ЖЙ«Ј©+2H+ЁTCr2O72-ЈЁіИЙ«Ј©+H2O
ЈЁ1Ј©ИфЖҪәвМеПөөДpH=12Ј¬ФтИЬТәПФ______Й«Ј®
ЈЁ2Ј©ДЬЛөГчөЪўЩІҪ·ҙУҰҙпЖҪәвЧҙМ¬өДКЗ______Ј®
aЈ®Cr2O72-өДЕЁ¶ИІ»ұд?bЈ®Cr2O72-әНCrO42-өДЕЁ¶ИПаН¬
cЈ®2vЈЁCr2O72-Ј©=vЈЁCrO42-Ј©?dЈ®ИЬТәөДСХЙ«І»ұд
ЈЁ3Ј©өЪўЪІҪ·ҙУҰЦРЈ¬»№ФӯјБОӘ______Ј¬ёГ»№ФӯјБ·ҙУҰәуЙъіЙ______Ј®
ЈЁ4Ј©өз¶Ж·ПТәЦРCr2O72-»№ҝЙНЁ№эПВБР·ҙУҰЧӘ»ҜЈә
Cr2O72-ЈЁaqЈ©+2Pb2+ЈЁaqЈ©+H2OЈЁlЈ©ЁT2PbCrO4ЈЁsЈ©+2H+ЈЁaqЈ©ЎчHЈј0
ёГ·ҙУҰҙпЖҪәвәуЈ¬ёДұдәбЧшұкұнКҫөД·ҙУҰМхјюЈ¬ПВБРКҫТвНјХэИ·өДКЗ______
ІОҝјҙр°ёЈәЈЁ1Ј©ИфЖҪәвМеПөөДpH=12Ј¬2CrO42-ЈЁ»ЖЙ«Ј©+2H+ЁTCr2O72-ЈЁіИЙ«Ј©+H2OДжПтТЖ¶ҜЈ¬ФтИЬТәОӘ»ЖЙ«Ј¬№Кҙр°ёОӘЈә»ЖЈ»
ЈЁ2Ј©ёщҫЭЕР¶ПЖҪәвЧҙМ¬өД·Ҫ·ЁЈәVХэ=VДжЈ¬»тёчЧй·ЦөДЕЁ¶ИұЈіЦІ»ұдФтЛөГчТСҙпЖҪәвЈ¬
aЈ®Cr2O72-өДЕЁ¶ИІ»ұдЈ¬ОӘМШХч¶ЁЈ¬ДЬЕР¶ПЖҪәвЈ¬№КaХэИ·Ј»?
bЈ®Cr2O72-әНCrO42-өДЕЁ¶ИПаН¬ИЎҫцУЪЖрКјЕЁ¶ИәНЧӘ»ҜЈ¬І»ДЬЕР¶ПЖҪәвЈ¬№КbҙнОуЈ»
cЈ®2vЈЁCr2O72-Ј©=vЈЁCrO42-Ј©Ј¬І»ДЬЕР¶ПХэДж·ҙУҰЛЩВК№ШПөЈ¬І»ДЬЕР¶ПЖҪәвЈ¬№КcҙнОуЈ»
dЈ®ИЬТәөДСХЙ«І»ұдЈ¬ОӘМШХч¶ЁЈ¬ДЬЕР¶ПЖҪәвЈ¬№КdХэИ·Ј»
№Кҙр°ёОӘЈәadЈ»
ЈЁ3Ј©өЪўЪІҪ·ҙУҰЦРЈ¬CrФӘЛШөД»ҜәПјЫҪөөНЈ¬ФтFeФӘЛШөД»ҜәПјЫЙэёЯЈ¬ЛщТФОӘFe2+Ј¬ұ»Сх»ҜОӘFe3+Ј¬№Кҙр°ёОӘЈәFe2+Ј»Fe3+Ј»
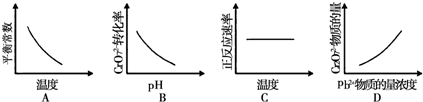
ЈЁ4Ј©AЈ®ЙэёЯОВ¶ИЖҪәвДжПтТЖ¶ҜЈ¬»ҜС§ЖҪәвіЈКэјхРЎЈ¬УлНјПуТ»ЦВЈ¬№КAХэИ·Ј»
BЈ®pHФҪҙуЈ¬ЖҪәвХэПтТЖ¶ҜөДЗчКЖФҪҙуЈ¬Cr2O72-ЧӘ»ҜВКФцҙуЈ¬УлНјПуІ»·ыЈ¬№КBҙнОуЈ»
CЈ®ЙэёЯОВ¶ИЈ¬·ҙУҰЛЩВКФцҙуЈ¬УлНјПуІ»·ыЈ¬№КCҙнОуЈ»
DЈ®Pb2+өДЕЁ¶ИФҪҙуЈ¬ЖҪәвХэПтТЖ¶ҜЈ¬Cr2O72-өДОпЦКөДБҝФҪРЎЈ¬УлНјПуІ»·ыЈ¬№КDҙнОуЈ»
№Кҙр°ёОӘЈәAЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв Ді№ӨТөЙъІъЦР·ўЙъПВБР·ҙУҰЈә2SO2ЈЁgЈ©+O2ЈЁgЈ©=2SO3ЈЁgЈ©Ј»ЎчHЈј0Ј®ПВБРУР№ШЛө·ЁХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®№ӨТөЙъІъSO3ІЙИЎёЯС№Ј¬ТтёЯС№УРАыУЪSO3ЙъіЙ
BЈ®№ӨТөТ»°гУГҪПёЯОВ¶ИәПіЙSO3Ј¬ТтёЯОВҝЙМбёЯSO2өДЧӘ»ҜВК
CЈ®ФЪПаН¬ОВ¶ИПВЈ¬УРјЧТТБҪөИМе»эИЭЖчЈ¬јЧЦРідИл2molSO2әН1molO2Ј¬ТТЦРідИл4molSO3Ј®ЖҪәвКұc1c2·ЦұрұнКҫјЧЎўТТЦРSO3¶ФУҰЕЁ¶ИЈ¬ФтУРc1Јјc2
DЈ®ІЙУГҙЯ»ҜјБЈ¬ТтІЙУГҙЯ»ҜјБҝЙМбёЯSO2өДЧӘ»ҜВК
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
3ЎўСЎФсМв ·ҙУҰЈәўЩPCl5ЈЁgЈ©?PCl3ЈЁgЈ©+Cl2ЈЁgЈ©?ўЪ2HIЈЁgЈ©?H2ЈЁgЈ©+I2ЈЁgЈ©?ўЫ2NO2ЈЁgЈ©?N2O4ЈЁgЈ©ФЪТ»¶ЁМхјюПВЈ¬ҙпөҪ»ҜС§ЖҪәвКұЈ¬·ҙУҰОпөДЧӘ»ҜВКҫщКЗ?a%Ј®ИфұЈіЦёч·ҙУҰөДОВ¶ИәНИЭЖчөДМе»э¶јІ»ёДұдЈ¬·ЦұрФЩјУИлТ»¶ЁБҝөДёчЧФөД·ҙУҰОпЈ¬ФтЧӘ»ҜВКЈЁЎЎЎЎЈ©
AЈ®ҫщІ»ұд
BЈ®ўЩФцҙуЈ¬ўЪІ»ұдЈ¬ўЫјхЙЩ
CЈ®ўЩјхЙЩЈ¬ўЪІ»ұдЈ¬ўЫФцҙу
DЈ®ҫщФцҙу
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
4ЎўСЎФсМв І»ДЬУГ»ҜС§ЖҪәвТЖ¶ҜФӯАнЛөГчөДКВКөКЗ
[? ]
AЈ®әПіЙ°ұФЪёЯС№ПВҪшРРКЗУРАыөД
BЈ®ОВ¶И№эёЯ¶ФәПіЙ°ұІ»Аы
CЈ®К№УГҙЯ»ҜјБДЬК№әПіЙ°ұЛЩВКјУҝм
DЈ®ј°Кұ·ЦАлҙУәПіЙЛюЦРіцАҙөД»мәПЖшЈ¬УРАыУЪәПіЙ°ұ
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ПВБРКВКөІ»ДЬУГАХПДМШБРФӯАнҪвКНөДКЗ
[? ]
AЈ®Ҫ«ВИ»ҜМъИЬТәјУИИХфёЙЧоЦХөГІ»өҪВИ»ҜМъ№ММе
BЈ®ёЦМъФЪіұКӘөДҝХЖшЦРИЭТЧЙъРв
CЈ®КөСйКТҝЙУГЕЕұҘәНКіСОЛ®өД·Ҫ·ЁКХјҜВИЖш
DЈ®іЈОВПВЈ¬Ҫ«1mLpH=3өДҙЧЛбИЬТәјУЛ®ПЎКНЦБl00mLЈ¬ІвөГЖдpH<5
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г