1ˇ˘ŃˇÔńĚâ ĎÂÁĐČÜҺһ¶¨łĘÖĐĐÔµÄĘÇŁ¨?Ł©
AŁ®c(H+)=10-7mol/LµÄČÜŇş
BŁ®pH=7µÄČÜŇş
CŁ®c(H+)/c(OH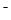 )=10-14µÄČÜŇş
)=10-14µÄČÜŇş
DŁ®°±Ë®ÓëÂČ»Żď§µÄ»ěşĎŇşÖĐc(NH4+)=c(Cl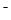 )
)
˛ÎżĽ´đ°¸ŁşD
±ľĚâ˝âÎöŁş
ŐýČ·´đ°¸ŁşD
Aˇ˘BÖ»ÓĐÔÚ25ˇćŁ¬KW=10¨D14ʱŁ¬˛ĹÓĐżÉÄÜłĘÖĐĐÔŁ¬Cˇ˘Ň»¶¨łĘĽîĐÔŁ¬Dˇ˘ÓɵçşÉĘŘşăc(H+)Ł«c(NH4Ł«)=c(OH¨D )Ł«c(Cl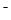 ), c(NH4+)=c(Cl
), c(NH4+)=c(Cl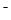 )ʱŁ¬c(H+)=c(OH
)ʱŁ¬c(H+)=c(OH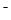 )
)
±ľĚâÄѶȣşĽňµĄ
2ˇ˘ŃˇÔńĚâ ŇŃÖŞŁş
Fe(s)+1/2O2(g)==FeO(s) ˇ÷H=-272 kJˇ¤mol-1
2Al(s)+3/2O2(g)==Al2O3 (s) ˇ÷H=-1675. 8 kJˇ¤mol-1
¸ůľÝŇÔÉĎČČ»ŻŃ§·˝łĚĘ˝Ł¬ĹжĎĎÂÁĐ˵·¨Öв»ŐýČ·µÄĘÇ
[? ]
AŁ®ÂÁµÄČĽÉŐČȡ÷H=-1675.8 kJˇ¤mol-1
BŁ®µČÖĘÁżµÄFe(s)ˇ˘Al(s)˝řĐĐÉĎĘö·´Ó¦Ł¬ÂÁ·ĹłöµÄČČÁż¶ŕ
CŁ®1 mol Al(s)Óë×ăÁżFeO(s)ÍęČ«·´Ó¦Ł¬·Ĺłö429.9 kJČČÁż
DŁ®1 mol Al2O3 (s)·Ö˝âłÉAl(s)şÍO2(g)µÄąýłĚÖĐŁ¬ĐčÎüĘŐ1675.8kJČČÁż
˛ÎżĽ´đ°¸ŁşC
±ľĚâ˝âÎöŁş
±ľĚâÄѶȣşŇ»°ă
3ˇ˘ŃˇÔńĚâ ĎÂÁĐČČ»ŻŃ§·˝łĚĘ˝ÖС÷H´ú±íČĽÉŐČȵÄĘÇŁ¨ˇˇˇˇŁ©
AŁ®C6H12O6?Ł¨sŁ©+6O2?Ł¨gŁ©=6CO2?Ł¨gŁ©+6H2O?Ł¨lŁ©ˇ÷H1
BŁ®S?Ł¨sŁ©+
O2?Ł¨gŁ©=SO3?Ł¨sŁ©ˇ÷H2
CŁ®CH4?Ł¨gŁ©+2O2Ł¨gŁ©=CO2?Ł¨gŁ©+2H2O?Ł¨gŁ©ˇ÷H3
DŁ®2CO?Ł¨gŁ©+O2Ł¨gŁ©=2CO2?Ł¨gŁ©ˇ÷H4
˛ÎżĽ´đ°¸ŁşAˇ˘ĆĎĚŃĚÇČĽÉŐÉúłÉ¶ţŃő»ŻĚĽĆřĚĺşÍҺ̬ˮËů·ĹłöµÄČČÁżłĆÎŞ¸ĂÎďÖʵÄČĽÉŐČČŁ¬ąĘAŐýČ·Ł»
Bˇ˘ÁňµĄÖĘČĽÉŐÉúłÉ¶ţŃő»ŻÁňËů·ĹłöµÄČČĽ´ÎŞČĽÉŐČČŁ¬ąĘB´íÎóŁ»
Cˇ˘Ľ×ÍéČĽÉŐÉúłÉ¶ţŃő»ŻĚĽĆřĚĺşÍҺ̬ˮËů·ĹłöµÄČČÁżłĆÎŞ¸ĂÎďÖʵÄČĽÉŐČČŁ¬ąĘC´íÎóŁ»
Dˇ˘ČČ»ŻŃ§·˝łĚĘ˝±íĘľµÄĘÇ1molżÉČĽÎďČĽÉŐ·ĹłöµÄČČÁżŁ¬ąĘD´íÎóŁ»
ąĘѡAŁ®
±ľĚâ˝âÎöŁş
±ľĚâÄѶȣşŇ»°ă
4ˇ˘ŃˇÔńĚâ ĽşÖŞŁşHCN(aq)ÓëNaOH(aq)·´Ó¦µÄˇ÷H="-12.1" kJ/molŁ»HCl(aq)ÓëNaOH(aq)·´Ó¦µÄˇ÷H=-55.6kJ/molˇŁÔňHCNÔÚË®ČÜŇşÖеçŔëµÄˇ÷HµČÓÚŁ¨?Ł©
AŁ®-67.7 kJ/mol
BŁ®-43.5 kJ/mol
CŁ®+43.5 kJ/mol
DŁ®+67.7 kJ/mol
˛ÎżĽ´đ°¸ŁşC
±ľĚâ˝âÎöŁş¸ůľÝÄÜÁżĘغ㶨ÂÉżÉÖŞŁ¬HCNÔÚË®ČÜŇşÖеçŔëµÄˇ÷HŁ˝-12.1 kJ/molŁ«55.6kJ/molŁ˝+43.5 kJ/molŁ¬´đ°¸ŃˇCˇŁ
±ľĚâÄѶȣşŇ»°ă
5ˇ˘ŃˇÔńĚâ ĎÂÁĐĐđĘöŐýČ·µÄĘÇŁ¨?Ł©
AŁ®pH=3şÍpH=5µÄŃÎËá¸÷10mL»ěşĎŁ¬ËůµĂČÜŇşµÄpH=4
BŁ®ČÜŇşÖĐcŁ¨H+Ł©Ô˝´óŁ¬pHֵҲԽ´óŁ¬ČÜŇşµÄËáĐÔľÍԽǿ
CŁ®ŇşÂČËäČ»˛»µĽµçŁ¬µ«ČÜ˝âÓÚË®şóµĽµçÇéżöÁĽşĂŁ¬Ňň´ËŁ¬ŇşÂČҲĘÇÇżµç˝âÖĘ
DŁ®µ±Î¶Ȳ»±äʱŁ¬ÔÚ´żË®ÖĐĽÓČëÇżĽîČÜŇş˛»»áÓ°ĎěË®µÄŔë×Ó»ýłŁĘý
˛ÎżĽ´đ°¸ŁşD
±ľĚâ˝âÎöŁşÂÔ
±ľĚâÄѶȣşĽňµĄ