1ЎўСЎФсМв ·ҙУҰA2(g) + 3B2(g)  ?4C2(g) + 2D2 (g)?ФЪ5LГЬұХИЭЖчЦРҪшРРЈ¬°л·ЦЦУәуЈ¬C2өДОпЦКөДБҝФцјУБЛ0.3molЈ¬ФтҙЛ·ҙУҰөДЖҪҫщЛЩВК
?4C2(g) + 2D2 (g)?ФЪ5LГЬұХИЭЖчЦРҪшРРЈ¬°л·ЦЦУәуЈ¬C2өДОпЦКөДБҝФцјУБЛ0.3molЈ¬ФтҙЛ·ҙУҰөДЖҪҫщЛЩВК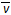 x
x
AЈ®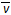 (B2) =" 0.01molЎӨ(LЎӨs)" ЁC1
(B2) =" 0.01molЎӨ(LЎӨs)" ЁC1
BЈ®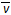 (C2) =" 0.002molЎӨ(LЎӨs)" ЁC1
(C2) =" 0.002molЎӨ(LЎӨs)" ЁC1
CЈ®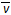 (D2) =" 0.006molЎӨ(LЎӨs)" ЁC1
(D2) =" 0.006molЎӨ(LЎӨs)" ЁC1
DЈ®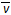 (A2) =" 0.008molЎӨ(LЎӨs)" ЁC1
(A2) =" 0.008molЎӨ(LЎӨs)" ЁC1
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәCөДОпЦКөДБҝЕЁ¶ИФцјУБЛ0.3mol/5L=0.06mol/LЈ¬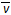 (C2) =" 0.06mol/30s=0.002molЎӨ(LЎӨs)" ЁC1?№КСЎB
(C2) =" 0.06mol/30s=0.002molЎӨ(LЎӨs)" ЁC1?№КСЎB
ұҫМвДС¶ИЈәјтөҘ
2ЎўСЎФсМв ФЪЛДёцІ»Н¬өДИЭЖчЦРЈ¬ФЪІ»Н¬МхјюПВҪшРР·ҙУҰЈәN2+3H2 2NH3ёщҫЭФЪПаН¬КұјдДЪІв¶ЁөДҪб№ыЕР¶ПЈ¬ЙъіЙ°ұЖшөДЛЩВКЧоҝмөДКЗ
2NH3ёщҫЭФЪПаН¬КұјдДЪІв¶ЁөДҪб№ыЕР¶ПЈ¬ЙъіЙ°ұЖшөДЛЩВКЧоҝмөДКЗ
AЈ®ҰФ(H2)=0Ј®2molЎӨL-1ЎӨs-1
BЈ®ҰФ(N2)=0Ј®4molЎӨL-1ЎӨmin-1
CЈ®ҰФ(NH3)=0Ј®3molЎӨL-1ЎӨs-1
DЈ®ҰФ(H2)=0Ј®6molЎӨL-1ЎӨmin-1
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә¶ФУЪ·ҙУҰN2ЈЁgЈ©+3H2ЈЁgЈ© 2NH3ЈЁgЈ©Ј¬ПИНіТ»өҘО»Ј¬И»әу¶јЧӘ»ҜОӘЗвЖшөД·ҙУҰЛЩВКҪшРРЕР¶ПЈ¬AЎўvЈЁH2Ј©="0.2" molЎӨL-1ЎӨs-1="12" molЎӨL-1ЎӨmin-1Ј»BЎўvЈЁN2Ј©=0.4mol/ЈЁL?minЈ©Ј¬·ҙУҰЛЩВКЦ®ұИөИУЪЖдјЖБҝКэЦ®ұИЈ¬ЛщТФvЈЁH2Ј©=3vЈЁN2Ј©="1.2" molЎӨL-1ЎӨmin-1Ј»CЎўvЈЁNH3Ј©="0.3" molЎӨL-1ЎӨs-1="18" molЎӨL-1ЎӨmin-1Ј¬·ҙУҰЛЩВКЦ®ұИөИУЪЖдјЖБҝКэЦ®ұИЈ¬ЛщТФvЈЁH2Ј©=1.5vЈЁNH3Ј©=27mol/ЈЁL?minЈ©Ј»DЎўvЈЁH2Ј©=0.6mol/ЈЁL?minЈ©Ј»СЎCЎЈ
2NH3ЈЁgЈ©Ј¬ПИНіТ»өҘО»Ј¬И»әу¶јЧӘ»ҜОӘЗвЖшөД·ҙУҰЛЩВКҪшРРЕР¶ПЈ¬AЎўvЈЁH2Ј©="0.2" molЎӨL-1ЎӨs-1="12" molЎӨL-1ЎӨmin-1Ј»BЎўvЈЁN2Ј©=0.4mol/ЈЁL?minЈ©Ј¬·ҙУҰЛЩВКЦ®ұИөИУЪЖдјЖБҝКэЦ®ұИЈ¬ЛщТФvЈЁH2Ј©=3vЈЁN2Ј©="1.2" molЎӨL-1ЎӨmin-1Ј»CЎўvЈЁNH3Ј©="0.3" molЎӨL-1ЎӨs-1="18" molЎӨL-1ЎӨmin-1Ј¬·ҙУҰЛЩВКЦ®ұИөИУЪЖдјЖБҝКэЦ®ұИЈ¬ЛщТФvЈЁH2Ј©=1.5vЈЁNH3Ј©=27mol/ЈЁL?minЈ©Ј»DЎўvЈЁH2Ј©=0.6mol/ЈЁL?minЈ©Ј»СЎCЎЈ
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ПВБРЛө·ЁЦРХэИ·өДКЗЈЁ?Ј©
AЈ®·ІКЗ·ЕИИ·ҙУҰ¶јКЗЧФ·ўөДЈ¬УЙУЪОьИИ·ҙУҰ¶јКЗ·ЗЧФ·ўөД
BЈ®ЧФ·ў·ҙУҰФЪЗЎөұМхјюПВІЕДЬКөПЦ
CЈ®ҙЯ»ҜјБІ»У°Пм·ҙУҰ»о»ҜДЬө«ДЬФцҙуөҘО»Ме»эДЪ»о»Ҝ·ЦЧУ°Щ·ЦКэЈ¬ҙУ¶шФцҙу·ҙУҰЛЩВК
DЈ®Фцҙу·ҙУҰОпЕЁ¶ИЈ¬ҝЙФцҙуөҘО»Ме»эДЪ»о»Ҝ·ЦЧУөД°Щ·ЦКэЈ¬ҙУ¶шК№УРР§ЕцЧІҙОКэФцҙу
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәAПоЈ¬»ҜС§·ҙУҰДЬ·сЧФ·ўҪшРРУЙЎчH+TЎчSҫц¶ЁЎЈAПоЈ¬ҙнОуЈ»BПоЈ¬ХэИ·ЎЈ ҙЯ»ҜјБНЁ№эУ°Пм·ҙУҰөД»о»ҜДЬҪш¶шФцҙуөҘО»Ме»эДЪөД»о»ҜДЬ·ЦЧУКэЈ¬У°Пм»ҜС§·ҙУҰЛЩВКЈ¬CПоЈ¬ҙнОуЎЈФцҙу·ҙУҰОпЕЁ¶ИЈ¬ҝЙФцҙуөҘО»Ме»эДЪ»о»Ҝ·ЦЧУөДёцКэЈ¬DПоҙнОуЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ·ҙУҰ4NH3(g)+5O2ЈЁgЈ©ЈҪ4NOЈЁgЈ©+6H2OЈЁgЈ©ФЪ2ЙэГЬұХИЭЖчЦРҪшРР1·ЦЦУәуЈ¬NH3јхЙЩБЛ0.12 molЈ¬ФтЖҪҫщГҝГлЦУЕЁ¶Иұд»ҜХэИ·өДКЗ
AЈ®H2OЈә0.002 molЎӨLЈӯ1
BЈ®NOЈә0.001 molЎӨLЈӯ1
CЈ®NH3Јә 0.002 molЎӨLЈӯ1
DЈ®O2Јә0.0025 molЎӨLЈӯ1