1ЎўСЎФсМв ФЪЛбРФИЬТәЦРДЬҙуБҝ№ІҙжЈ¬ІўЗТИЬТәОӘОЮЙ«НёГчөДАлЧУЧйКЗ
AЈ®Na+ЎўK+ЎўSiO32ЈӯЎўSO42Јӯ
BЈ®NH4+ЎўNa+ЎўNO3ЈӯЎўClЈӯ
CЈ®K+ЎўCa2+ЎўHCO3ЈӯЎўClЈӯ
DЈ®Mg2+ЎўK+ЎўClЈӯЎўMnO4ЁD
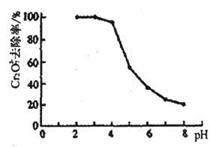
2ЎўМоҝХМв ЈЁ10·ЦЈ©ЛжЧЕ№ӨТөөДСёЛЩ·ўХ№Ј¬ІъЙъөД·ПЛ®¶ФЛ®МеөДОЫИҫТІИХЗчСПЦШЎЈНЁ№эҝШЦЖИЬТәөДpH¶Ф№ӨТө·ПЛ®ЦРөДҪрКфАлЧУҪшРР·ЦАлКЗКөјК№ӨЧчЦРҫӯіЈК№УГөД·Ҫ·ЁЎЈПВұнКЗіЈОВПВҪрКфЗвСх»ҜОпөДKsp(іБөнИЬҪвЖҪәвіЈКэ)әНҪрКфАлЧУФЪДіЕЁ¶ИПВҝӘКјіБөнЛщРиөДpH(ұнЦРЕЁ¶ИОӘПаУҰpHКұИЬТәЦРУР№ШҪрКфАлЧУІъЙъіБөнөДЧоРЎЕЁ¶ИЈ»өұИЬТәЦРҪрКфАлЧУЕЁ¶ИРЎУЪ10-5 mol?L-1КұНЁіЈИПОӘёГАлЧУіБөнНкИ«)ЎЈ
ҪрКфАлЧУ
| Ksp
| pH(10-1 mol?L-1)?
| ?pH(10-5 mol?L-1)
|
?Fe3+
| ?4.0ЎБ10-38
| ?2.7
| ?3.7
|
Cr3+ ?
| 6.0ЎБ10-31
| 4.3
| 5.6
|
Cu2+
| 2.2ЎБ10-20
| ?4.7
| 6.7
|
Ca2+
| ?4.0ЎБ10-5
| ?12.3?
| ?14.3
|
(1)Діі§ЕЕіцөД·ПЛ®ЦРә¬УРCu2+әНFe3+Ј¬ІвөГЖдЕЁ¶ИҫщРЎУЪ0.1 mol?L-1ЎЈОӘіэИҘЖдЦРөДFe3+Ј¬»ШКХНӯЈ¬РиҝШЦЖөДpH·¶О§КЗ_______________________________ЎЈ
(2)ОӘБЛҙҰАнә¬УРCr2O72-ЛбРФИЬТәөД№ӨТө·ПЛ®Ј¬ІЙУГИзПВ·Ҫ·ЁЈәПт·ПЛ®ЦРјУИЛККБҝNaClЈ¬ТФFeОӘөзј«ҪшРРөзҪвЈ¬ҫӯ№эТ»¶ОКұјдЈ¬УРCr(OH)3әНFe(OH)3іБөнЙъіЙЕЕіцЈ¬ҙУ¶шК№·ПЛ®ЦРёхә¬БҝөНУЪЕЕ·ЕұкЧјЎЈ
ўЩCr2O72-ЧӘұдОӘCr3+өДАлЧУ·ҪіМКҪОӘ______________________ЎЈ
ўЪpH¶Ф·ПЛ®ЦРCr2O72-ИҘіэВКөДУ°ПмИзНјЎЈДгИПОӘөзҪв№эіМЦРИЬТәөДpHИЎЦөФЪ______·¶О§ДЪ¶ФҪөөН·ПЛ®ЦРөДёхә¬БҝЧоУРАыЈ¬ЗлЛөГчАнУЙЈә_________________________________ЎЈ
[ЧўЈәИҘіэВК(ЈҘ)=[(c0-c)ЈҜco]ЎБ100ЈҘЈ¬КҪЦРЈәcoЎӘҙҰАнЗ°·ПЛ®ЦРCr2O72-ЕЁ¶ИЈ¬cЎӘҙҰАнәу·ПЛ®ЦРCr2O72-өДЕЁ¶И]
(3)іБөнЧӘ»ҜФЪЙъІъЦРТІУРЦШТӘУҰУГЎЈАэИзЈ¬УГNa2CO3ИЬТәҝЙТФҪ«№шВҜЛ®№ёЦРөДCaSO4ЧӘ»ҜОӘҪПКиЛЙ¶шТЧЗеіэөДCaCO3Ј¬ёГіБөнЧӘ»ҜҙпөҪЖҪәвКұЈ¬ЖдЖҪәвіЈКэKЈҪ_________(РҙКэЦө)ЎЈ[ТСЦӘKsp (CaSO4)=9.1x10-6Ј¬Ksp (CaCO3)=2.8ЎБ10-9]
3ЎўСЎФсМв ФЪПВБРёчИЬТәЦРЈ¬АлЧУТ»¶ЁДЬҙуБҝ№ІҙжөДКЗ?ЈЁ?Ј©?
AЈ®ЗҝјоРФИЬТәЦРЈә 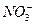 Ўў
Ўў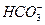 Ўў
Ўў Ўў
Ўў
BЈ®ә¬УР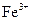 өДИЬТәЦРЈә
өДИЬТәЦРЈә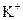 Ўў
Ўў ЎўH+ЎўAlO2-
ЎўH+ЎўAlO2-
CЈ®ДіОЮЙ«ИЬТәЦРЈә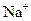 ЎўCu2+ЎўSO42-Ўў
ЎўCu2+ЎўSO42-Ўў
DЈ®КТОВПВЈ¬pH=1өДИЬТәЦРЈә Ўў
Ўў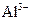 Ўў
Ўў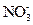 Ўў
Ўў
4ЎўМоҝХМв ЈЁ10·ЦЈ©ДіҙэІвИЬТәЈЁСфАлЧУОӘNaЈ«Ј©ЦРҝЙДЬә¬УРSO42ЈӯЎўSO32ЈӯЎўNO3ЈӯЎўClЈӯЎўBrЈӯЎўCO32ЈӯЎўHCO3ЈӯЦРөДТ»ЦЦ»т¶аЦЦЈ¬ҪшРРИзНјЛщКҫөДКөСйЈ¬ГҝҙОКөСйЛщјУКФјБҫщ№эБҝЈ¬»ШҙрТФПВОКМвЈә
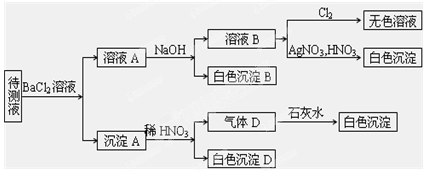
ЈЁ1Ј©ҙэІвТәЦРКЗ·сә¬УРSO42ЈӯЎўSO32ЈӯЈә_____________________ ЎЈ
ЈЁ2Ј©іБөнBөД»ҜС§КҪОӘ_________Ј»ЙъіЙіБөнBөДАлЧУ·ҪіМКҪОӘ
___________________ __ЎЈ
ЈЁ3Ј©ёщҫЭТФЙПКөСйЈ¬ҙэІвТәЦРҝП¶ЁГ»УРөДАлЧУКЗ___________________; ҝП¶ЁҙжФЪөДАлЧУ»№УР________________________ЎЈ
5ЎўСЎФсМв ДіИЬТәЦРҙжФЪҪП¶аөДOHЎӘЎўK+ЎўCO32ЎӘЈ¬ёГИЬТәЦР»№ҝЙДЬҙуБҝҙжФЪөДКЗ
AЈ®H+
BЈ®Ca2+
CЈ®NH4+
DЈ®SO42ЎӘ