1ЎўМоҝХМв ЈЁ12·ЦЈ©ФЪ450ЎжІўУРҙЯ»ҜјБҙжФЪПВЈ¬УЪТ»ИЭ»эәг¶ЁөДГЬұХИЭЖчДЪҪшРРПВБР·ҙУҰЈә
2SO2(g)Ј«O2(g) 2SO3(g)?ЎчHЈҪЁD190 kJЎӨmolЎӘ1
2SO3(g)?ЎчHЈҪЁD190 kJЎӨmolЎӘ1
ўЕёГ·ҙУҰ500ЎжКұөДЖҪәвіЈКэ________450ЎжКұөДЖҪәвіЈКэЈЁМоЎ°ЈҫЎұЎўЎ°ЈјЎұ»тЎ°ЈҪЎұЈ©ЎЈ
ўЖЕР¶ПёГ·ҙУҰҙпөҪЖҪәвЧҙМ¬өДұкЦҫКЗ_______________ЎЈЈЁМоЧЦДёЈ©
aЈ®SO2әНSO3ЕЁ¶ИПаөИ? bЈ®SO2°Щ·Цә¬БҝұЈіЦІ»ұд
cЈ®ИЭЖчЦРЖшМеөДС№ЗҝІ»ұд? dЈ®v(O2)ХэЈҪ2v(SO3)Дж
eЈ®ИЭЖчЦР»мәПЖшМеөДГЬ¶ИұЈіЦІ»ұд
ўЗУыМбёЯSO2өДЧӘ»ҜВКЈ¬ПВБРҙлК©ҝЙРР өДКЗ_______________ЎЈЈЁМоЧЦДёЈ©
өДКЗ_______________ЎЈЈЁМоЧЦДёЈ©
aЈ®ПтЧ°ЦГЦРФЩідИлN2? bЈ®ПтЧ°ЦГЦРФЩідИлO2
cЈ®ёДұд·ҙУҰөДҙЯ»ҜјБ? dЈ®ЙэёЯОВ¶И
ўИФЪТ»ёц№М¶ЁИЭ»эОӘ5 LөДГЬұХИЭЖчЦРідИл0.20 mol SO2әН0.10 mol O2Ј¬°л·ЦЦУәуҙпөҪЖҪәвЈ¬ІвөГИЭЖчЦРә¬SO3 0.18 molЈ»ИфјМРшНЁИл0.20 mol SO2әН0.10 mol O2Ј¬ФтЖҪәв_________________ТЖ¶ҜЈЁМоЎ°ПтХэ·ҙУҰ·ҪПтЎұЎўЎ°ПтДж·ҙУҰ·ҪПтЎұ»тЎ°І»ЎұЈ©Ј¬ФЩҙОҙпөҪЖҪәвәуЈ¬______ molЈјn(SO3)Јј______molЎЈ
ІОҝјҙр°ёЈә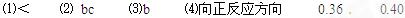
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ¶ФУЪҝЙДж·ҙУҰ? 2AB3(g)
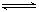 ?A2(g) Ј« 3B2(g) ; ҰӨHЈҫ0Ј¬ПВБРНјПсХэИ·өДКЗ
?A2(g) Ј« 3B2(g) ; ҰӨHЈҫ0Ј¬ПВБРНјПсХэИ·өДКЗ
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г
3ЎўМоҝХМв
ўс. јУИл3mol SO2әН2mol O2·ўЙъ·ҙУҰЈ¬ҙпөҪЖҪәвКұ·ЕіцөДИИБҝОӘ ____________
ўт. ұЈіЦН¬Т»·ҙУҰОВ¶ИЈ¬ФЪПаН¬ИЭЖчЦРЈ¬Ҫ«ЖрКјОпЦКөДБҝёДОӘ? a mol SO2?Ўў b mol O2 Ўўc mol SO3 (g) Ј¬УыК№ЖҪәвКұSO3өДМе»э·ЦКэОӘ2/9ЎЈ
ўЕҙпөҪЖҪәвКұЈ¬ўсәНўт·ЕіцөДИИБҝ ______ЈЁМоРтәЕЈ©
AЈ®ЎӘ¶ЁПаөИ? BЈ®З°ХЯТ»¶ЁРЎ? C. З°ХЯҙуУЪ»төИУЪәуХЯ
(2) aЎўbЎўcұШРлВъЧгөД№ШПөКЗ(Т»ёцУГaЎўcұнКҫЈ¬БнТ»ёцУГbЎўcұнКҫ)________________
ІОҝјҙр°ёЈәўЗ УыК№ЖрКј·ҙУҰұнПЦОӘПтХэ·ҙУҰ·ҪПтҪшРРЈ¬a өДИЎЦө·¶О§КЗ____________________ўс? 98.3KJ?ўтўЕ C?ўЖa+c="3?" 2b+c="4?" ўЗ 2<aЎЬ3
ұҫМвҪвОцЈәұҫМвОӘ»ҜС§ЖҪәвәН·ҙУҰИИЧЫәПКФМвЈ¬ОӘҪПДСКФМвЎЈ
ўсЎўУЙМвТвЖшМеС№ЗҝОӘЖрК№КұөД90%Ј¬Фт·ҙУҰәуЧЬөДОпЦКөДБҝОӘЖрК№КұөД90%Ј¬Йи·ҙУҰ№эіМЦРO2ПыәДөДОпЦКөДБҝОӘxЈ¬ФтЖҪәвКұЧЬОпЦКөДБҝОӘЈЁ5-xЈ©Ј¬ЈЁ5-xЈ©=4.5molЦӘx=0.5molјҙ·ҙУҰБЛөДO2ОӘ0.5molЈ¬ҙЛКұ·ЕіцөДИИБҝОӘ196.6KJЁM2= 98.3KJЎЈН¬КұҝЙЗуіцSO3өДМе»э·ЦКэОӘ2/9ЎЈ
ўтЎўЖҪәвКұSO3өДМе»э·ЦКэТІОӘ2/9Ј¬УЙўсЦӘБҪХЯОӘөИР§ЖҪәвЎЈўЕИфcОӘБгЈ¬Фт·ЕіцөДИИБҝБҪХЯПаөИЈ¬ИфcІ»ОӘБгЈ¬Фт·ЕіцөДИИБҝЗ°ХЯҙуУЪәуХЯЎЈ(2)УЙөИР§ЖҪәвЦРөИБҝөИР§№ШПөІ»ДСХТіцa+c="3?" 2b+c=4№ШПөЈ»(3)УЙј«Цө·ЁҝЙЗу2<aЎЬ3ЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ФЪТ»ёцІ»өјИИөДГЬұХ·ҙУҰЖчЦР,Ц»·ўЙъБҪёц·ҙУҰ:
a(g)+b(g) 2c(g)ЎЎҰӨH1<0
2c(g)ЎЎҰӨH1<0
x(g)+3y(g) 2z(g)ЎЎҰӨH2>0
2z(g)ЎЎҰӨH2>0
ҪшРРПа№ШІЩЧчЗТҙпөҪЖҪәвәу(әцВФМе»эёДұдЛщЧцөД№Ұ),ПВБРРрКцҙнОуөДКЗ(ЎЎЎЎ)
AЈ®өИС№Кұ,НЁИл¶иРФЖшМе,cөДОпЦКөДБҝІ»ұд
BЈ®өИС№Кұ,НЁИлzЖшМе,·ҙУҰЖчЦРОВ¶ИЙэёЯ
CЈ®өИИЭКұ,НЁИл¶иРФЖшМе,ёч·ҙУҰЛЩВКІ»ұд
DЈ®өИИЭКұ,НЁИлzЖшМе,yөДОпЦКөДБҝЕЁ¶ИФцҙу
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәұҫМвТӘМШұрЧўТвМвёЙЦРөДРЕПўЎ°І»өјИИөДГЬұХ·ҙУҰЖчЎұЎЈAПо,өИС№Кұ,НЁИл¶иРФЖшМе,ЖшМеөДМе»эФцҙу,ЖҪәвx(g)+3y(g) 2z(g)(ҰӨH2>0)ПтЧуТЖ¶Ҝ,·ҙУҰ·ЕИИ,·ҙУҰМеПөөДОВ¶ИЙэёЯ,УЙУЪёГ·ҙУҰИЭЖчКЗТ»ёцІ»өјИИөДИЭЖч,ЛщТФЖҪәвa(g)+b(g)
2z(g)(ҰӨH2>0)ПтЧуТЖ¶Ҝ,·ҙУҰ·ЕИИ,·ҙУҰМеПөөДОВ¶ИЙэёЯ,УЙУЪёГ·ҙУҰИЭЖчКЗТ»ёцІ»өјИИөДИЭЖч,ЛщТФЖҪәвa(g)+b(g) 2c(g)ТІПтЧу(ОьИИ·ҪПт)ТЖ¶Ҝ,cөДОпЦКөДБҝјхРЎ,№КAІ»ХэИ·;BПо,өИС№Кұ,НЁИлzЖшМе,ФцҙуБЛЙъіЙОпөДЕЁ¶И,ЖҪәвx(g)+3y(g)
2c(g)ТІПтЧу(ОьИИ·ҪПт)ТЖ¶Ҝ,cөДОпЦКөДБҝјхРЎ,№КAІ»ХэИ·;BПо,өИС№Кұ,НЁИлzЖшМе,ФцҙуБЛЙъіЙОпөДЕЁ¶И,ЖҪәвx(g)+3y(g) 2z(g)ПтЧуТЖ¶Ҝ,УЙУЪёГ·ҙУҰөДДж·ҙУҰКЗ·ЕИИ·ҙУҰ,ЛщТФ·ҙУҰЖчөДОВ¶ИЙэёЯ,BПоХэИ·;өИИЭКұ,НЁИл¶иРФЖшМе,ёч·ҙУҰОпәНЙъіЙОпөДОпЦКөДБҝГ»УРұд»Ҝ,јҙёчЧй·ЦөДЕЁ¶ИГ»УР·ўЙъұд»Ҝ,ЛщТФёчЧй·ЦөД·ҙУҰЛЩВКІ»·ўЙъұд»Ҝ,CПоХэИ·;өИИЭКұ,НЁИлzЖшМе,ФцҙуБЛЙъіЙОпzөДЕЁ¶И,ЖҪәвДжПтТЖ¶Ҝ,ЛщТФyөДОпЦКөДБҝЕЁ¶ИФцҙу,DПоХэИ·ЎЈ
2z(g)ПтЧуТЖ¶Ҝ,УЙУЪёГ·ҙУҰөДДж·ҙУҰКЗ·ЕИИ·ҙУҰ,ЛщТФ·ҙУҰЖчөДОВ¶ИЙэёЯ,BПоХэИ·;өИИЭКұ,НЁИл¶иРФЖшМе,ёч·ҙУҰОпәНЙъіЙОпөДОпЦКөДБҝГ»УРұд»Ҝ,јҙёчЧй·ЦөДЕЁ¶ИГ»УР·ўЙъұд»Ҝ,ЛщТФёчЧй·ЦөД·ҙУҰЛЩВКІ»·ўЙъұд»Ҝ,CПоХэИ·;өИИЭКұ,НЁИлzЖшМе,ФцҙуБЛЙъіЙОпzөДЕЁ¶И,ЖҪәвДжПтТЖ¶Ҝ,ЛщТФyөДОпЦКөДБҝЕЁ¶ИФцҙу,DПоХэИ·ЎЈ
ұҫМвДС¶ИЈәТ»°г
5ЎўМоҝХМв БтөвСӯ»··ЦҪвЛ®ЦЖЗвЦчТӘЙжј°ПВБР·ҙУҰЈә
I SO2+2H2O+I2=H2SO4+2HI
II 2HI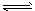 H2+I2
H2+I2
ўу 2H2SO4=2SO2+O2+2H2O
ЈЁ1Ј©·ЦОцЙПКц·ҙУҰЈ¬ПВБРЕР¶ПХэИ·өДКЗ____ЎЈ
aЈ®·ҙУҰўуТЧФЪіЈОВПВҪшРР
bЈ®·ҙУҰIЦРSO2Сх»ҜРФұИHIЗҝ
cЈ®Сӯ»·№эіМЦРРиІ№ідH2O
dЈ®Сӯ»·№эіМІъЙъ1molO2өДН¬КұІъЙъ1mol H2
ЈЁ2Ј©Т»¶ЁОВ¶ИПВЈ¬Пт1LГЬұХИЭЖчЦРјУИл1molHI(g)Ј¬·ўЙъ ·ҙУҰIIЈ¬H2ОпЦКөДБҝЛжКұјдөДұд»ҜИзУТНјЛщКҫЎЈ0~2 minДЪөДЖҪҫщ·ҙУҰЛЩВКv(HI)=____ЎЈёГОВ¶ИПВөДЖҪәвіЈКэK=____ ПаН¬ОВ¶ИПВЈ¬ИфҝӘКјјУИлHI(g)өДОпЦКөДБҝКЗФӯАҙөД2ұ¶Ј¬Фт___КЗФӯАҙөД2ұ¶ЎЈ
aЈ®ЖҪәвіЈКэ
bЈ®HIөДЖҪәвЕЁ¶И
cЈ®ҙпөҪЖҪәвөДКұјд
dЈ®ЖҪәвКұH2өДМе»э·ЦКэ

ЈЁ3Ј©КөСйКТУГZnәНПЎБтЛбЦЖИЎH2Ј¬·ҙУҰКұИЬТәЦРЛ®өДөзАлЖҪәв___ТЖ¶ҜЈЁМо Ў°ПтЧуЎұЎ°ПтУТЎұ»тЎ°І»ЎұЈ©Ј»ИфјУИлЙЩБҝПВБРКФјБЦРөД____Ј¬ІъЙъH2өДЛЩВКҪ«ФцҙуЎЈ
aЈ®NaNO3
bЈ®CuSO4
cЈ®Na2SO4
dЈ®NaHSO3
ЈЁ4Ј©ТФH2ОӘИјБПҝЙЦЖЧчЗвСхИјБПөзіШЎЈТСЦӘ2H2(g)+O2(g) = 2H2O(1) ЎчH=-572kJЎӨmol-1 ДіЗвСхИјБПөзіШКН·Е228.8 kJөзДЬКұЈ¬ЙъіЙ1molТәМ¬Л®Ј¬ёГөзіШөДДЬБҝЧӘ»ҜВКОӘ________ЎЈ
ІОҝјҙр°ёЈәЈЁ1Ј©c
ЈЁ2Ј©0.1 mol.L-1ЎӨmin-1 Ј»64Ј»b
ЈЁ3Ј©ПтУТЈ»b Ўўf
ЈЁ4Ј©80%
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г