1ЎўСЎФсМв ПВБРОпЦККфУЪЗҝөзҪвЦКөДКЗЈЁ?Ј©
AЈ®CH3COOH
BЈ®H2O
CЈ®AgNO3
DЈ®NH3ЎӨH2O
2ЎўСЎФсМв ПВБР»ҜС§УГУпКйРҙХэИ·өДКЗ
A.ЙЩБҝNa2SO3ИЬТәөОИлNaClOИЬТәЦРАлЧУ·ҪіМКҪЈәH2O+2ClO-+SOЁTCl2Ўь+SO+2OH-
B.ЗвСхИјБПөзіШЈЁKOHЧчөзҪвЦКИЬТәЈ©өДХэј«·ҙУҰКҪ 2H2O+2e-ЁTH2Ўь+2OH-
C.ёЦМъ·ўЙъОьСхёҜКҙөДёәј«·ҙУҰКҪЈәFe-3e-ЁTFe3+
D.іОЗеКҜ»ТЛ®УлЙЩБҝРЎЛХҙтИЬТә»мәПЈәCa2++OH-+HCOЁTCaCO3Ўэ+H2O
3ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪІ»ХэИ·өДКЗ
A.ҙЧЛбјУИл°ұЛ®ЈәCH3COOH+NH3?H2OCH3COO-+NH4++H2O
B.НӯЖ¬ІеИлПхЛбТшИЬТәЈәCu+Ag+=Cu2++Ag
C.МјЛбёЖјУИлҙЧЛбЈәCO32-+2CH3COOH=CO2Ўь+2CH3COO-+H2O
D.БтЗи»ҜјШИЬТәјУИлИэВИ»ҜМъИЬТәЈәFe3++SCN-=[FeЈЁSCNЈ©]2+
4ЎўСЎФсМв ДЬЛөГчҙЧЛбКЗИхөзҪвЦКөДКВКөКЗ
[? ]
AЈ®ҙЧЛбИЬТәөДөјөзРФұИСОЛбЗҝ
BЈ®ҙЧЛбИЬТәУлМјЛбёЖ·ҙУҰЈ¬»әВэ·Еіц¶юСх»ҜМј
CЈ®ҙЧЛбИЬТәУГЛ®ПЎКНәуЈ¬H+ЕЁ¶ИПВҪө
DЈ®0. 1molЎӨL-1өДҙЧЛбИЬТәЦРЈ¬c(H+)ФјОӘ0. 001molЎӨL-1
5ЎўМоҝХМв іЈОВПВЈ¬Ҫ«ДіТ»ФӘЛбHAәНNaOHИЬТәөИМе»э»мәПЈ¬БҪЦЦИЬТәөДЕЁ¶ИәН»мәПәуЛщөГИЬТәөДpHИзПВұн
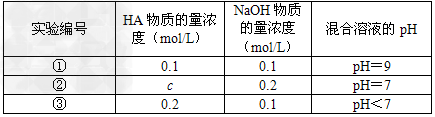
Зл»ШҙрЈә
ЈЁ1Ј©ҙУўЩЧйЗйҝц·ЦОцЈ¬HAКЗЗҝЛб»№КЗИхЛбЈҝ____________ЈЁМоЎ°ЗҝЛбЎұ»тЎ°ИхЛбЎұЈ©
ЈЁ2Ј©ўЪЧйЗйҝцұнГчЈ¬c____________0.2?mol/LЈЁСЎМоЎ°ҙуУЪЎұЎўЎ°РЎУЪЎұ»тЎ°өИУЪЎұЈ©ЎЈ»мәПТәЦРАлЧУЕЁ¶ИcЈЁAЈӯЈ©УлcЈЁNaЈ«Ј©өДҙуРЎ№ШПөКЗ____________ЎЈ
ЈЁ3Ј©ҙУўЫЧйКөСйҪб№ы·ЦОцЈ¬ЛөГчHAөДөзАліМ¶И_________NaAөДЛ®ҪвіМ¶ИЈЁСЎМоЎ°ҙуУЪЎұЎўЎ°РЎУЪЎұ»тЎ°өИУЪЎұЈ©Ј¬ёГ»мәПИЬТәЦРАлЧУЕЁ¶ИУЙҙуөҪРЎөДЛіРтКЗ____________ЎЈ
ЈЁ4Ј©ўЩЧйКөСйЛщөГ»мәПИЬТәЦРУЙЛ®өзАліцөДcЈЁOHЈӯЈ©ЈҪ____________molЎӨLЈӯ1ЎЈ