1ЎўјтҙрМв ТСЦӘЈәCO2+3H2O+2AlO2-ЁT2AlЈЁOHЈ©3Ўэ+CO32-Ј®ПЦУРЕЁ¶ИОӘ0.1mol?L-1өДОеЦЦөзҪвЦКИЬТәЈәўЩNa2CO3?ўЪNaHCO3ўЫNaAlO2ўЬCH3COONa?ўЭNaOH
ЈЁ1Ј©ХвОеЦЦИЬТәөДpHУЙРЎөҪҙуөДЛіРтКЗ______ЈЁМоұаәЕЈ©Ј»
ЈЁ2Ј©Ҫ«ОеЦЦИЬТәПЎКНПаН¬өДұ¶КэКұЈ¬ЖдpHұд»ҜЧоҙуөДКЗ______ЈЁМоұаәЕЈ©Ј»
ЈЁ3Ј©»мәПМјЛбЈЁH2CO3Ј©ИЬТәәНNaAlO2ИЬТәЈ¬КФРҙіцҝЙДЬ·ўЙъөД»ҜС§·ҙУҰ·ҪіМКҪЈә______Ј»
ЈЁ4Ј©іЈОВПВЈ¬Ҫ«ДіТ»ФӘЛбHAәНNaOHИЬТәөИМе»э»мәПЈ¬БҪЦЦИЬТәөДЕЁ¶ИәН»мәПәуЛщөГИЬТәөДpHИзПВұнЈә
| КөСйұаәЕ | HAОпЦКөДБҝЕЁ¶ИЈЁmol/LЈ© | NaOHОпЦКөДБҝЕЁ¶ИЈЁmol/LЈ© | »мәПИЬТәөДpH
јЧ
0.20
0.20
pH=a
ТТ
0.10
0.10
pH=8.00
|
І»ҝјВЗТТЧйөДКөСйҪб№ыЈ¬өҘҙУјЧЧйЗйҝц·ЦОцЈ¬ИзәОУГaЈЁ»мәПИЬТәөДpHЈ©АҙЛөГчHAКЗЗҝЛб»№КЗИхЛб______Ј»
ТТЧйКөСйЛщөГ»мәПИЬТәЦРУЙЛ®өзАліцөДc?ЈЁOH-Ј©=______mol/LЈ®
ЗуіцёГ»мәПИЬТәЦРПВБРЛгКҪөДЦөЈә
IЈ®c?ЈЁNa+Ј©-c?ЈЁA-Ј©=______Ј»
IIЈ®c?ЈЁOH-Ј©-c?ЈЁHAЈ©=______Ј®
ІОҝјҙр°ёЈәЈЁ1Ј©ЈәўЬЎўўЪЎўўЩЎўўЫКЗСОЈ¬ўЭКЗјоЈ¬ОпЦКөДБҝЕЁ¶ИПаН¬өДХвјёЦЦИЬТәЈ¬СОөДPHЦөРЎУЪјоөДЈ¬ЛщТФўЭөДPHЦөЧоҙуЈ»ўЬЎўўЪЎўўЩЎўўЫЛДЦЦСОөДСфАлЧУПаН¬Ј¬ЖдТхАлЧУПаУҰөДЛбФҪИхЈ¬СОөДPHЦөФҪҙуЈ¬ҙЧЛбөДЛбРФЈҫМјЛбөДЛбРФЈҫЖ«ВБЛбөДЛбРФЈ¬МјЛбЗвДЖКЗЛбКҪСОЈ¬МјЛбДЖКЗХэСОЈ¬ЛщТФМјЛбДЖөДPHЦөҙуУЪМјЛбЗвДЖөДЈ¬№КПаН¬ОпЦКөДБҝЕЁ¶ИөДХвјёЦЦИЬТәөДPHЦөУЙРЎөҪҙуөДЛіРтКЗўЬўЪўЩўЫўЭЈ®
№Кҙр°ёОӘЈәўЬўЪўЩўЫўЭЈ®
?ЈЁ2Ј©ўЬўЪўЩўЫ¶јҙжФЪЛ®ҪвЖҪәвЈ¬өұПЎКНКұЈ¬СОУЦЛ®ҪвіцІҝ·ЦАлЧУҪшРРІ№ідЈ»ЗвСх»ҜДЖКЗЗҝјоЈ¬НкИ«өзАлЈ¬І»ҙжФЪөзАлЖҪәвЈ¬ЛщТФөұПЎКНКұЈ¬ЖдpHұд»ҜЧоҙуЈ®
№Кҙр°ёОӘЈәўЭЈ®
ЈЁ3Ј©МјЛбөДЛбРФҙуУЪЖ«ВБЛбөДЛбРФЈ¬МјЛбДЬәНЖ«ВБЛбДЖ·ҙУҰЙъіЙМјЛбЗвДЖәНЗвСх»ҜВБЈ¬
NaAlO2+H2CO3+H2O=NaHCO3+AlЈЁOHЈ©3ЎэЈ»МјЛбЗвДЖДЬөзАліцЗвАлЧУЈ¬ЗвАлЧУәНЖ«ВБЛбДЖ·ҙУҰЙъіЙМјЛбДЖәНЗвСх»ҜВБ
NaHCO3+NaAlO2+H2O=Na2CO3+AlЈЁOHЈ©3ЎэЈ®
№Кҙр°ёОӘЈәNaAlO2+H2CO3+H2O=NaHCO3+AlЈЁOHЈ©3ЎэЎўNaHCO3+NaAlO2+H2O=Na2CO3+AlЈЁOHЈ©3ЎэЈ®
ЈЁ4Ј©ЛбЎўјоөДОпЦКөДБҝПаөИЈ¬јҙЛбЎўјоЗЎәГ·ҙУҰЙъіЙСОЈ¬ИфСОИЬТәөДa=7КұЈ¬ёГСОКЗЗҝЛбЗҝјоСОЈ¬HAКЗЗҝЛбЈ»aЈҫ7КұЈ¬ёГСОКЗЗҝјоИхЛбСОЈ¬HAКЗИхЛбЈ»
?ТТЧйКөСйЈәPH=8Ј¬ЛщТФCЈЁH+Ј©=10-8 mol/LЈ¬ёщҫЭCЈЁH+Ј©Ј®CЈЁOH-Ј©=10-14Ј¬ЛщТФc?ЈЁOH-Ј©=10-6 mol/LЈ»
ўсёщҫЭөзәЙКШәгЦӘЈ¬CЈЁH+Ј©+CЈЁNa+ Ј©=CЈЁOH-Ј©+CЈЁA- Ј©Ј¬ЛщТФc?ЈЁNa+Ј©-c?ЈЁA-Ј©=CЈЁOH-Ј©-CЈЁH+Ј©=ЈЁ10-6-10-8Ј©mol/LЈ®
ўтёщҫЭОпБПКШәгәНөзәЙКШәгјЖЛгЈ¬CЈЁH+Ј©+CЈЁNa+ Ј©=CЈЁOH-Ј©+CЈЁA- Ј©Ј¬CЈЁA- Ј©+CЈЁHAЈ©=0.05mol/LЈ¬
CЈЁNa+ Ј©=0.05mol/L?
ЛщТФc?ЈЁOH-Ј©-c?ЈЁHAЈ©=CЈЁH+Ј©=10-8 mol/LЈ®
№Кҙр°ёОӘЈәa=7КұЈ¬HAКЗЗҝЛбЈ»aЈҫ7КұЈ¬HAКЗИхЛбЈ»ЈЁ10-6-10-8Ј©mol/LЈ»=10-8 mol/LЈ¬Ј»
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ФЪХф·ўГуЦРјУИИХфёЙПВБРОпЦКөДИЬТәІўЧЖЙХЈ¬ҝЙөГФӯУРОпЦКөД№ММеКЗЈЁ ? Ј©
AЈ®ВИ»ҜВБ
BЈ®МјЛбЗвГҫ
CЈ®БтЛбСЗМъ
DЈ®МјЛбДЖ
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
3ЎўМоҝХМв ЈЁ8·ЦЈ©РҙіцПВБРОпЦКөДөзАл·ҪіМКҪЈә
NaOH??Na2CO3??H2SO4??NH4NO3?
ІОҝјҙр°ёЈә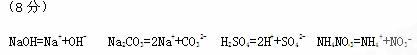
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ
4ЎўСЎФсМв ПВБРОпЦКЦРЈ¬І»ДЬөзАліцЛбёщАлЧУөДКЗЈЁ ? Ј©
AЈ®Na2S
BЈ®BaЈЁOHЈ©2
CЈ®KMnO4
DЈ®KCl
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
5ЎўСЎФсМв ПВБРЛө·ЁХэИ·өДКЗЈЁ?Ј©
AЈ®ВИ»ҜДЖИЬТәФЪөзБчЧчУГПВөзАліЙNa+УлCl?
BЈ®өзҪвЦКөзАліцөДХэөзәЙЧЬКэУлёәөзәЙЧЬКэТ»¶ЁПаөИ
CЈ®ФЪЛ®ИЬТәАпДЬөзАліцТхЎўСфАлЧУөД»ҜәПОпіЖОӘСО
DЈ®ИЬУЪЛ®әуДЬөзАліцH+өД»ҜәПОп¶јКЗЛб
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәВИ»ҜДЖИЬТәКЗФЪЛ®·ЦЧУөДЧчУГПВ·ўЙъөДөзАлЈ¬№КAПоҙнОуЈ»BПоХэИ·Ј»CПоЦРФЪОпЦКЛ®ИЬТәАпЦ»ТӘДЬөзАлФтТ»¶ЁөзАліцТхЎўСфАлЧУЈ¬ёГОпЦКҝЙДЬКЗЛб»тјо»тСОЈ¬№КёГПоҙнОуЈ»ИЬУЪЛ®әуДЬөзАліцөДСфАлЧУИ«ІҝКЗH+өД»ҜәПОпІЕКЗЛбЈ¬№КDПоҙнОуЎЈ
ұҫМвДС¶ИЈәјтөҘ