1ЎўјтҙрМв ДіРЈС§ЙъФЪКөСйКТЦРЙијЖБЛИзПВЧ°ЦГЈ¬ДЈДвәоөВ°сЦЖјо·ЁЈ¬»сөГМјЛбЗвДЖҫ§МеЈ¬·ҙУҰФӯАнҝЙУГИзПВ»ҜС§·ҪіМКҪұнКҫЈәNH3+CO2+NaCl+H2OЁTNH4Cl+NaHCO3ЎэЈ¬ТАҫЭҙЛФӯАнЈ¬ФЩҪ«МјЛбЗвДЖҫ§МејУИИ·ЦҪв¶шЦЖөГМјЛбДЖҫ§МеЈ¬ЖдЦРBЧ°ЦГЦРөДКФ№ЬДЪКЗИЬУР°ұәНВИ»ҜДЖөДИЬТәЈ¬ЗТ¶юХЯҫщТСҙпөҪұҘәНЈә
ЈЁ1Ј©AЧ°ЦГөДГыіЖКЗЈә______Ј®CЧ°ЦГЦРПЎБтЛбөДЧчУГОӘЈә______Ј¬К№УГЗтРОёЙФп№ЬөДДҝөДКЗЈә______Ј®
ЈЁ2Ј©ПВұнЦРЛщБРіцөДКЗПа№ШОпЦКФЪІ»Н¬ОВ¶ИПВөДИЬҪв¶ИКэҫЭЈЁg/100gЛ®Ј©
| 0Ўж | 10Ўж | 20Ўж | 30Ўж | 40Ўж | 50Ўж
NaCl
35.7
35.8
36.0
36.3
36.6
37.0
NaHCO3
6.9
8.1
9.6
11.1
12.7
14.5
NH4Cl
29.4
33.3
37.2
41.4
45.8
50.4
|
ІОХХұнЦРКэҫЭЈ¬Зл·ЦОцBЧ°ЦГЦРК№УГұщЛ®КЗТтОӘ______Ј®
ЈЁ3Ј©ёГРЈС§ЙъФЪјмІйНкҙЛМЧЧ°ЦГЖшГЬРФәуҪшРРКөСйЈ¬Ҫб№ыГ»УРөГөҪМјЛбЗвДЖҫ§МеЈ¬ЦёөјҪМКҰЦёіцУҰФЪ______Ч°ЦГЦ®јдЈЁМоРҙЧЦДёЈ©Б¬ҪУТ»ёцКўУР______өДПҙЖшЧ°ЦГЈ¬ЖдЧчУГКЗ______Ј®
ЈЁ4Ј©ИфёГРЈС§ЙъҪшРРКөСйКұЈ¬ЛщУГұҘәНКіСОЛ®ЦРә¬NaClөДЦКБҝОӘ5.85gЈ¬КөСйәуөГөҪёЙФпөДNaHCO3ҫ§МеөДЦКБҝОӘ5.04gЈ¬ФтNaHCO3өДІъВКОӘ______Ј®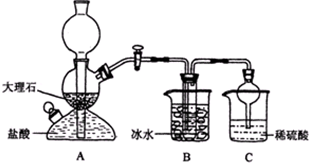
ІОҝјҙр°ёЈәЈЁ1Ј©ТАҫЭЧ°ЦГНј·ЦОцЈ¬AОӘЖфЖХ·ўЙъЖчЈ¬УГАҙЦЖИЎ№ММеәНТәМеІ»РиТӘјУИИЈ¬ЙъіЙөДЖшМеДСИЬУЪЛ®өДЖшМеЦЖұё·ҙУҰЈ¬ТАҫЭәоөВ°сЦЖјо·ЁЈ¬ҝЙЦӘЙъіЙ¶юСх»ҜМјЈ»ЖдЦРBЧ°ЦГЦРөДКФ№ЬДЪКЗИЬУР°ұәНВИ»ҜДЖөДИЬТәЈ¬ЗТ¶юХЯҫщТСҙпөҪұҘәНЈ»CЧ°ЦГЦРөДПЎБтЛбКұОьКХ»У·ўіцөД°ұЖшЈ¬ұЬГвОЫИҫҝХЖшЈ»ТтОӘ°ұЖшј«ТЧИЬУЪЛ®Ј¬ЗтРОёЙФп№ЬөДЧйЧ°КЗОӘБЛ·АЦ№ө№ОьЈ¬
№Кҙр°ёОӘЈәЖфЖХ·ўЙъЖчЈ»ОьКХҙУBЧ°ЦГЦРөДКФ№ЬДЪТЭіцөД°ұЖшЈ¬јхЙЩОЫИҫЈ»·АЦ№ө№ОьЈ»
ЈЁ2Ј©НјұнЦРОпЦКИЬҪв¶ИұИҪПҝЙЦӘЈ¬ОВ¶ИФҪөНЈ¬МјЛбЗвДЖөДИЬҪв¶ИФҪРЎЈ¬ұгУЪОціцЈ¬·ыәПЦЖұёТӘЗ󣬹Кҙр°ёОӘЈәОВ¶ИФҪөНЈ¬МјЛбЗвДЖөДИЬҪв¶ИФҪРЎЈ¬ұгУЪОціцЈ»
ЈЁ3Ј©јмІйНкҙЛМЧЧ°ЦГЖшГЬРФәуҪшРРКөСйЈ¬Ҫб№ыГ»УРөГөҪМјЛбЗвДЖҫ§МеЈ¬ЛөГчЙъіЙөД¶юСх»ҜМјЖшМеЦРә¬УРВИ»ҜЗвЈ¬әН°ұЖш·ҙУҰәуЈ¬ИЬТәЦРІ»ТЧЙъіЙМјЛбЗвДЖЈ»РиТӘФЪAәНBЦ®јдАыУГұҘәНМјЛбЗвДЖИЬТәіэИҘВИ»ҜЗвЈ¬И»әуНЁИл°ұ»ҜөДұҘәНКіСОЛ®ЦРЈ¬·ҙУҰОціцМјЛбЗвДЖҫ§МеЈ¬№Кҙр°ёОӘЈәAУлBЈ»ұҘәНNaHCO3ИЬТәЈ»іэИҘCO2ЦР»мәПөДHClЖшМеЈ»
ЈЁ4Ј©ЛщУГұҘәНКіСОЛ®ЦРә¬NaClөДЦКБҝОӘ5.85gЈ¬КөСйәуөГөҪёЙФпөДNaHCO3ҫ§МеөДЦКБҝОӘ5.04gЈ¬ТАҫЭ»ҜС§·ҙУҰЈәNaCl+H2O+NH3+CO2=NaHCO3Ўэ+NH4ClЈ»5.85gNaClИ«Іҝ·ҙУҰЙъіЙNaHCO3ЦКБҝОӘ8.4gЈ¬КөјКЙъіЙ5.04gЈ¬ЛщТФNaHCO3өДІъВК=КөјКБҝАнВЫБҝЎБ100%=5.04g8.4gЎБ100%=60%Ј¬
№Кҙр°ёОӘЈә60%Ј®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўМоҝХМв МјЛбДЖКЗФмЦҪЎўІЈБ§Ўў·ДЦҜЎўЦЖёпөИРРТөөДЦШТӘФӯБПЎЈ№ӨТөМјЛбДЖ(ҙҝ¶ИФј98%)ЦРә¬УРCa2+ЎўMg2+ЎўFe3+ ЎўCl-әНSO42-өИФУЦКЈ¬Мбҙҝ№ӨТХВ·ПЯИзПВЈә
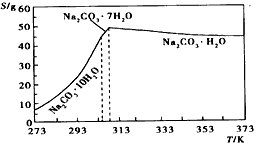
ТСЦӘМјЛбДЖөДИЬҪв¶И(S)ЛжОВ¶Иұд»ҜөДЗъПЯИзПВНјЛщКҫЈә
»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ВЛФьөДЦчТӘіЙ·ЦОӘ____ЎЈ
ЈЁ2Ј©Ў°іГИИ№эВЛЎұөДФӯТтКЗ____ЎЈ
ЈЁ3Ј©ИфФЪКөСйКТҪшРРЎ°іГИИ№эВЛЎұЈ¬ҝЙІЙИЎөДҙлК©КЗ____ЈЁРҙіц1ЦЦЈ©ЎЈ
ЈЁ4Ј©ИфЎ°ДёТәЎұСӯ»·К№УГЈ¬ҝЙДЬіцПЦөДОКМвј°ЖдФӯТтКЗ____ЎЈ
ЈЁ5Ј©ТСЦӘЈә
Na2CO3ЎӨl0H2O(s)=Na2CO3(s) +10H2O(g) ЎчH1= +532. 36kJЎӨmol-1
Na2CO3ЎӨ10H2O(s)=Na2CO3ЎӨH2O(s) +9H2O(g) ЎчH2= +473.63kJЎӨmol-1
РҙіцNa2CO3ЎӨH2OНСЛ®·ҙУҰөДИИ»ҜС§·ҪіМКҪ__________________________________ЎЈ
ІОҝјҙр°ёЈәЈЁ1Ј©Fe(OH)3ЎўCaCO3әНMg(OH)2
ЈЁ2Ј©·АЦ№ОВ¶ИПВҪөКұNa2CO3ЎӨH2OИЬҪвЈ¬ұЬГвОціцNa2CO3ЎӨ10H2OЈ¬»тNa2CO3ЎӨ7H2O
ЈЁ3Ј©ФӨИИ№эВЛЧ°ЦГ
ЈЁ4Ј©ІъЖ·ҙҝ¶ИҪөөНЈ¬ТтОӘСӯ»·К№УГКұДёТәЦРCl-УлSO42-АЫ»эЈ¬өјЦВІъЖ·ә¬»тNa2SO4ФУЦК
ЈЁ5Ј©Na2CO3ЎӨH2O==Na2CO3+H2O ЎчH=+58.73KJ/mol
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
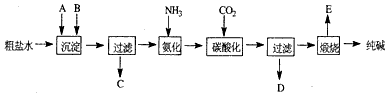
3ЎўМоҝХМв №ӨТөЙъІъҙҝјоөД№ӨТХБчіМКҫТвНјИзПВ
НкіЙПВБРМоҝХ
ЈЁ1Ј©ҙЦСОЛ®јУИліБөнјБAЎўBіэФУЦКЈЁіБөнјБAАҙФҙУЪКҜ»ТТӨі§Ј©Ј¬РҙіцAЎўBөД»ҜС§КҪЎЈ
A_____________B____________
ЈЁ2Ј©КөСйКТМбҙҝҙЦСОөДКөСйІЩЧчТАҙООӘЈәИЎСщЎў________ЎўіБөнЎў_________Ўў__________ЎўАдИҙҪбҫ§Ўў_________ЎўәжёЙЎЈ
ЈЁ3Ј©№ӨТөЙъІъҙҝјо№ӨТХБчіМЦРЈ¬МјЛб»ҜКұІъЙъөДПЦПуКЗ____________ЎЈМјЛб»ҜКұГ»УРОціцМјЛбДЖҫ§МеЈ¬ЖдФӯТтКЗ____________ЎЈ
ЈЁ4Ј©МјЛб»Ҝәу№эВЛЈ¬ВЛТәDЧоЦчТӘөДіЙ·ЦКЗ____________ЈЁМоРҙ»ҜС§КҪЈ©Ј¬јмСйХвТ»іЙ·ЦөДТхАлЧУөДҫЯМе·Ҫ·ЁКЗЈә______________________________ЎЈ
ЈЁ5Ј©°ұјо·ЁБчіМЦР°ұКЗСӯ»·К№УГөДЈ¬ОӘҙЛЈ¬ВЛТәDјУИлКҜ»ТЛ®ІъЙъ°ұЎЈјУКҜ»ТЛ®әуЛщ·ўЙъөД·ҙУҰөДАлЧУ·ҪіМКҪОӘЈә_____________________ВЛТәDјУКҜ»ТЛ®З°ПИТӘјУИИЈ¬ФӯТтКЗ________________________ЎЈ
ЈЁ6Ј©ІъЖ·ҙҝјоЦРә¬УРМјЛбЗвДЖЎЈИз№ыУГјУИИ·ЦҪвөД·Ҫ·ЁІв¶ЁҙҝјоЦРМјЛбЗвДЖөДЦКБҝ·ЦКэЈ¬ҙҝјоЦРМјЛбЗвДЖөДЦКБҝ·ЦКэҝЙұнКҫОӘЈә__________________ЈЁЧўГчДгөДұнҙпКҪЦРЛщУГөДУР№Ш·ыәЕөДә¬ТеЈ©
ІОҝјҙр°ёЈәЈЁ1Ј©Ca(OH)2»тCaOЈ»Na2CO3
ЈЁ2Ј©ИЬҪвЈ»№эВЛЈ»Хф·ўЈ»№эВЛ
ЈЁ3Ј©УРҫ§МеОціцЈЁ»тіцПЦ»лЧЗЈ©Ј»МјЛбДЖИЬҪв¶ИұИМјЛбЗвДЖҙу
ЈЁ4Ј©NH4ClЈ»ИЎСщЈ¬јУПхЛбЛб»ҜЈ¬јУПхЛбТшЈ¬УР°ЧЙ«іБөнЈ¬ёГТхАлЧУКЗВИАлЧУ
ЈЁ5Ј©NH4++OH-==NH3Ўь+H2OЈ»·АЦ№јУКҜ»ТЛ®КұІъЙъМјЛбёЖіБөн
ЈЁ6Ј© ЈЁm1ЈәјУИИЗ°ҙҝјоөДЦКБҝЎўm2ЈәјУИИәуҙҝјоөДЦКБҝЈ©
ЈЁm1ЈәјУИИЗ°ҙҝјоөДЦКБҝЎўm2ЈәјУИИәуҙҝјоөДЦКБҝЈ©
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
4ЎўМоҝХМв ҙҝјоКЗТ»ЦЦЦШТӘөД»Ҝ№ӨФӯБПЎЈДҝЗ°ЦЖјо№ӨТөЦчТӘУРЎ°°ұјо·ЁЎұәНЎ°БӘәПЦЖ јо·ЁЎұБҪЦЦ№ӨТХЎЈЗл°ҙТӘЗу»ШҙрОКМвЈә
?ЈЁ1Ј©Ў°°ұјо·ЁЎұІъЙъҙуБҝCaCl2·ПЖъОпЈ¬ЗлРҙіцёГ№ӨТХЦРІъЙъCaCl2өД»ҜС§·ҪіМКҪЈә_______________________________________________________Ј»
ЈЁ2Ј©РҙіцЎ°БӘәПЦЖјо·ЁЎұУР№Ш·ҙУҰөД»ҜС§·ҪіМКҪЈә____________________________________________Ј»
ЈЁ3Ј© CO2КЗЦЖјо№ӨТөөДЦШТӘФӯБПЈ¬Ў°БӘәПЦЖјо·ЁЎұУлЎ°°ұјо·ЁЎұЦРCO2өДАҙФҙУРәОІ»Н¬Јҝ__________________________________________________________Ј»
ЈЁ4Ј©ВМЙ«»ҜС§өДЦШТӘФӯФтЦ®Т»КЗМбёЯ·ҙУҰөДФӯЧУАыУГВКЎЈёщҫЭЎ°БӘәПЦЖјо·ЁЎұЧЬ·ҙУҰЈ¬БРіцјЖЛгФӯЧУАыУГВКөДұнҙпКҪЈә
ФӯЧУАыУГВК(%) =________________________________ЎЈ
ІОҝјҙр°ёЈә
ЈЁ1Ј©2NH4Cl+Ca( OH)2===2NH3Ўь+CaCl2+ 2H2O?
ЈЁ2Ј©NH3+CO2 +H2O +NaClЈЁұҘәНЈ©=== NaHCO3Ўэ+NH4Cl? 2NaHCO3===Na2CO3 +CO2Ўь+H2O (»тРҙЧЬ·ҙУҰ·ҪіМКҪЈә2NaCl+2NH3 +CO2 +H2O===Na2CO3+ 2NH4C1)
ЈЁ3Ј©Ў°°ұјо·ЁЎұЦРөДCO2АҙФҙУЪКҜ»ТКҜмСЙХЈ¬Ў°БӘәПЦЖјо·ЁЎұЦРөДCO2АҙФҙУЪәПіЙ°ұ№ӨТөөД·ПЖшЈ»
ЈЁ4Ј©Ў°БӘәПЦЖјо·ЁЎұФӯЧУАыУГВКөДұнҙпКҪЈә

ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўМоҝХМв ЈЁИэСЎТ»Ј©Ўҫ»ҜС§УлјјКхЎҝ
ҙҝјоТ»ЦұТФАҙ¶јКЗ№ӨТөЙъІъөДЦШТӘФӯБПЈ¬әЬіӨТ»¶ОКұјдҙҝјоөДЦЖ·Ё¶јұ»Е·ГА№ъјТЛщВў¶ПЎЈЙПёцКАјНіхЈ¬ОТ№ъЦшГыөД№ӨТө»ҜС§јТәоөВ°сПИЙъҫӯ№эКэДкөД·ҙёҙСРҫҝЦХУЪ·ўГчБЛУЕУЪЕ·ГАЦЖјојјКхөДБӘәПЦЖјо·ЁЈЁУЦіЖәоКПЦЖјо·ЁЈ©ЎЈІўФЪМмҪтҪЁФмБЛОТ№ъ¶АБўСР·ўөДөЪТ»јТЦЖјоі§ЎЈЖдЦЖјоФӯАнөДБчіМИзНј
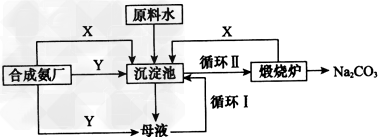
(1)әоөВ°сСЎФсМмҪтЧчОӘЦЖјоі§өДі§Ц·УРәОұгАыМхјюЈЁҫЩ¶юАэЛөГчЈ©Јә______Ўў_____ЎЈ
(2)әПіЙ°ұ№Өі§РиТӘПтЦЖјоі§МṩБҪЦЦФӯБПЖшМеЈ¬ЛьГЗ·ЦұрКЗ ____Ўў____ЈЁМо»ҜС§КҪЈ©Ј»ХвБҪЦЦЖшМеФЪК№УГ№эіМЦРКЗ·сРиТӘҝјВЗМнјУөДЛіРтЈә____ЈЁМоЎ°КЗЎұ»тЎ°·сЎұЈ©Ј¬ФӯТтКЗ____________________ЎЈ
(3)ФЪіБөніШЦР·ўЙъөД·ҙУҰөД»ҜС§·ҪіМКҪЈә________________ЎЈ
(4)К№ФӯБПЛ®ЦРИЬЦКөДАыУГВКҙУ70%МбёЯөҪ90%ТФЙПЈ¬ЦчТӘКЗЙијЖБЛ__________ЈЁМоЙПКцБчіМЦРөДұаәЕЈ©ЎЈҙУДёТәЦРҝЙТФМбИЎөДёұІъЖ·өДУҰУГКЗ____________ЈЁҫЩТ»АэЈ©ЎЈ
ІОҝјҙр°ёЈә(1)ФӯБП·бё»Ј»ФЛКдұгАы
(2)CO2Ј»NH3Ј»КЗЈ»°ұЖшФЪЛ®ЦРИЬҪв¶ИҙуЈ¬ПИНЁ°ұЖшәуНЁCO2ІъЙъМјЛбЗв臨࣬УРАыУЪМјЛбЗвДЖОціц
(3)NaCl(ұҘәН)+CO2+NH3+H2O=NaHCO3Ўэ+NH4Cl
(4)Сӯ»·IЈ»Чч»Ҝ·К
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г