1ЎўСЎФсМв ПтЛб»Ҝ№эөДMnSO4ИЬТәЦРөОјУ(NH4)2S2O8(№э¶юБтЛбп§)ИЬТә»б·ўЙъ·ҙУҰЈәMn2Ј«Ј«S2O82-Ј«H2OЁDЎъMnO4-Ј«HЈ«Ј«SO42-ЎЈПВБРЛө·ЁІ»ХэИ·өДКЗ(ЎЎЎЎ)
AЈ®ҝЙТФАыУГёГ·ҙУҰјмСйMn2Ј«
BЈ®Сх»ҜРФұИҪПЈәS2O82-ЈҫMnO4-
CЈ®MnSO4ИЬТәҝЙТФК№УГСОЛбЛб»Ҝ
DЈ®ИфУР0.1 molСх»ҜІъОпЙъіЙЈ¬ФтЧӘТЖөзЧУ0.5 mol
2ЎўКөСйМв ЈЁ15·ЦЈ©ОӘБЛМҪҫҝH2O2ЎўH2SO3әНBr2Сх»ҜРФөДПа¶ФЗҝИхЈ¬ЙијЖИзПВКөСйЈЁјРіЦТЗЖчТСВФИҘЈ©ЎЈЗл»ШҙрПВБРОКМвЈә
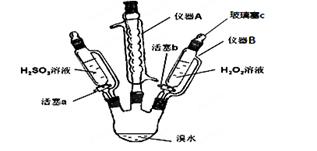
ЈЁ1Ј©ТЗЖчAөДГыіЖ_________Ј¬ЖдЧчУГКЗ___________ЎЈ
ЈЁ2Ј©УГТЗЖчBөОјУТәМеІўІ»РиТӘҙтҝӘІЈБ§ИыcЈ¬ФӯТтКЗ____________________________ЎЈ
ЈЁ3Ј©КөСйјЗВјИзПВЈЁЗлІ№И«ҝХ°ЧЈ©Јә
ІҪЦи
| КөСйІЩЧч
| КөСйПЦПу
| КөСйҪбВЫ
|
ўс
| ҙтҝӘ»оИыaЈ¬ЦрөОјУИлH2SO3ИЬТәЦБ№эБҝ
| ________________
| __________________________
|
ўт
| ПтІҪЦиўсЛщөГИЬТәЦРЦрөОјУИлH2O2ИЬТә
| ёХҝӘКјИЬТәСХЙ«ОЮГчПФұд»ҜЈ¬јМРшөОјУЈ¬ИЬТәұдОӘіИ»ЖЙ«
| __________________________
|
ЈЁ4Ј©ІҪЦиўтЦРЈ¬ҝӘКјКұСХЙ«ОЮГчПФұд»ҜөДФӯТтКЗЈЁРҙіцТ»МхЈ©_______________________Ј¬
ІҪЦиўсЦР·ҙУҰөДАлЧУ·ҪіМКҪ_________________________________________________Ј¬
ІҪЦиўтЦРЦчТӘ·ҙУҰөДАлЧУ·ҪіМКҪ_____________________________________________ЎЈ
3ЎўСЎФсМв ФЪЕЁСОЛбЦРH3AsO3УлSnCl2·ҙУҰөДАлЧУ·ҪіМ3SnCl2+12Cl-+2H3AsO3+6H+=2As+3SnCl62-+6MЈ®№ШУЪёГ·ҙУҰөДЛө·ЁЦРХэИ·өДЧйәПКЗЈЁЎЎЎЎЈ©ўЩСх»ҜјБКЗH3AsO3Ј»ўЪ»№ФӯРФЈәCl-ЈҫAsЈ»ўЫГҝЙъіЙ0.1mol?AsЈ¬·ҙУҰЦРЧӘТЖөДөзЧУөДОпЦКөДБҝОӘ0.3?molЈ®ўЬMОӘOH-Ј®
AЈ®ўЩўЫ
BЈ®ўЩўЪўЬ
CЈ®ўЪўЫўЬ
DЈ®Ц»УРўЩ
4ЎўСЎФсМв ҙУКҜУўЙ°ЦЖИЎІў»сөГёЯҙҝ№иөДЦчТӘ»ҜС§·ҙУҰИзПВЈә
ўЩSiO2Ј«2C Si(ҙЦ)Ј«2COЎь
Si(ҙЦ)Ј«2COЎь
ўЪSi(ҙЦ)Ј«2Cl2 SiCl4
SiCl4
ўЫSiCl4Ј«2H2 Si(ҙҝ)Ј«4HCl
Si(ҙҝ)Ј«4HCl
№ШУЪЙПКц·ҙУҰөД·ЦОцІ»ХэИ·өДКЗ
[? ]
AЈ®ўЩЎўўЫКЗЦГ»»·ҙУҰЈ¬ўЪКЗ»ҜәП·ҙУҰ
BЈ®ёЯОВПВЈ¬Ҫ№МҝУлЗвЖшөД»№ФӯРФҫщЗҝУЪ№и
CЈ®ИОТ»·ҙУҰЦРЈ¬ГҝПыәД»тЙъіЙ28?g№иЈ¬ҫщЧӘТЖ4?molөзЧУ
DЈ®ёЯОВПВҪ«КҜУўЙ°ЎўҪ№МҝЎўВИЖшЎўЗвЖш°ҙТ»¶ЁұИАэ»мәПҝЙөГёЯҙҝ№и
5ЎўСЎФсМв ТСЦӘЈәўЩ2FeCl3+2KI=2FeCl2+2KCl+I2ўЪ2FeCl2+Cl2=2FeCl3Ј¬ФтПВБРОўБЈ»№ФӯДЬБҰУЙҙуөҪРЎөДЛіРтХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®Fe2+ЈҫCl-ЈҫI-
BЈ®I-ЈҫFe2+ЈҫCl-
CЈ®I-ЈҫCl-ЈҫFe2+
DЈ®Cl-ЈҫI-ЈҫFe2+