1ЎўСЎФсМв ТСЦӘЈәKClO3+6HClЈЁЕЁЈ©ЁTKCl+3Cl2Ўь+3H2OЈ®ИзНјЛщКҫЈ¬Ҫ«ЙЩБҝКФјБ·Цұр·ЕИлЕаСшГуЦРөДПаУҰО»ЦГЈ¬КөСйКұҪ«ЕЁСОЛбөОФЪKClO3ҫ§МеЙПЈ¬ІўУГұнГжГуёЗәГЈ®ПВұнЦРУЙКөСйПЦПуөГіцөДҪбВЫНкИ«ХэИ·өДКЗЈЁЎЎЎЎЈ©

AЈ®A
BЈ®B
CЈ®C
DЈ®D
ІОҝјҙр°ёЈәAЎўҪ«ЕЁСОЛбөОФЪKClO3ҫ§МеЙПЈ¬УРCl2ЙъіЙЈ¬Cl2ҫЯУРЗҝСх»ҜРФЈ¬ДЬСх»ҜFeCl2ЙъіЙFeCl3Ј¬№КAҙнОуЈ»
BЎўCl2УлЛ®·ҙУҰЙъіЙHClәНҫЯУРЗҝСх»ҜРФөДHClOЈ¬ДЬСх»Ҝ·УМӘ¶шК№·УМӘИЬТәНКЙ«Ј¬№КBҙнОуЈ»
CЎўCl2УлЛ®·ҙУҰЙъіЙHClәНҫЯУРЗҝСх»ҜРФөДHClOЈ¬ИЬТәіКЛбРФІўҫЯУРЗҝСх»ҜРФЈ¬ДЬК№КҜИпКФТәПИұдәмәуНКЙ«Ј¬ұнПЦіцHClOөДЖҜ°ЧРФЈ¬№КCҙнОуЈ»
DЎўCl2ҫЯУРЗҝСх»ҜРФЈ¬УлKI·ҙУҰЙъіЙI2Ј¬өн·ЫИЬТәұдА¶Ј¬№КDХэИ·Ј®
№КСЎDЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ
2ЎўКөСйМв ЈЁ10·ЦЈ©ОӘҪІКЪЎ°ВИЖшөДРФЦКәНУГНҫЎұЈ¬ДіАПКҰАыУГ¶юСх»ҜГМәНЕЁСОЛбОӘЦчТӘФӯБПЈ¬ЙијЖТ»МЧИзНјЛщКҫөДКөСйЧ°ЦГ(ЖдЦРAКЗБ¬УРЧўЙдЖчХлН·өД ПрЖӨ№ЬЈ¬ХлН·ТСІеИлІўҙ©№эПрЖӨИы)ҪшРРҪМС§ЎЈКФ»ШҙрПВБРОКМвЈә
ПрЖӨ№ЬЈ¬ХлН·ТСІеИлІўҙ©№эПрЖӨИы)ҪшРРҪМС§ЎЈКФ»ШҙрПВБРОКМвЈә
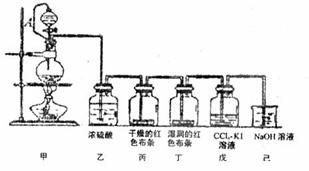
ЈЁ1Ј©јЧЦРЙХЖҝ·ўЙъ·ҙУҰөД»ҜС§·ҪіМКҪОӘ?ЎЈ
ЈЁ2Ј©ұыЦРөДПЦПуКЗ?Ј»
јәЦРөДАлЧУ·ҪіМКҪЈә______________________________Ј»ЎЈ
ЈЁ3Ј©АыУГПрЖӨ№ЬҪ«·ЦТәВ©¶·ЙП¶ЛУлЙХЖҝБ¬НЁЈ¬ЖдЧчУГКЗ?ЎЈ
ЈЁ4Ј©ТСЦӘВИЖшУлАдЛ®·ҙУҰөД»ҜС§·ҪіМОӘCl2 + H20 =" HCl" +? HClOЎЈ
ўЩДгИПОӘК№УРЙ«ІјМхНКЙ«өДОпЦККЗ?ЎЈ
ўЪОӘМҪҫҝЖдЙъіЙОпөДЖҜ°ЧРФ,ЗлЙијЖјтөҘөДКөСйјУТФЦӨГчЈ¬РҙіцКөСйөДІЩЧч·Ҫ·ЁЎўПЦПуәНҪбВЫ
ІОҝјҙр°ёЈәЈЁ10·ЦЈ©(1) 4HCl(ЕЁ)+Mn02 ==MnCl2+C12Ўь+2H20?ЈЁ2·ЦЈ©

ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ
3ЎўСЎФсМв ·ЕФЪіЁҝЪИЭЖчЦРөДПВБРИЬТәЈ¬ҫГЦГәуИЬТәЦРИЬЦКөДЕЁ¶И»бұдҙуөДКЗЈЁЈ©
AЈ®ЕЁБтЛб
BЈ®ЗвСх»ҜДЖ
CЈ®ЕЁСОЛб
DЈ®ВИ»ҜДЖ
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәЕЁH2SO4ТтОьЛ®¶шұдПЎЈ»NaOHФЪҝХЖшЦРТтұдЦКіЙNa2CO3өјЦВNaOHөДЕЁ¶ИПВҪөЈ»HClТЧ»У·ў¶шЕЁ¶ИПВҪөЈ»NaClИЬТәТтЛ®·Ц»У·ў¶шөјЦВЕЁ¶ИұдҙуЎЈ
ұҫМвДС¶ИЈәјтөҘ
4ЎўКөСйМв ЈЁ10·ЦЈ©ПВНјКЗКөСйКТЦЖИЎІўКХјҜCl2өДЧ°ЦГЎЈAКЗCl2·ўЙъЧ°ЦГЈ¬Ј¬EКЗУІЦКІЈБ§№ЬЦРЧ°УРПёМъЛҝНшЈ»FОӘёЙФпөД№гҝЪЖҝЈ¬ЙХұӯGОӘОІЖшОьКХЧ°ЦГЎЈ
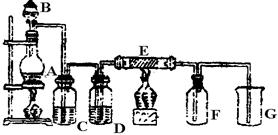
КФ»ШҙрЈә
ЈЁ1Ј©AЦР·ўЙъөД»ҜС§·ҙУҰ·ҪіМКҪОӘ?ЎЈ
ЈЁ2Ј©CЎўDЎўGЦРёчЧ°өДТ©Ж·ЈәC__________Ј»D__________Ј»G__________ЎЈ
ЈЁ3Ј©EЦРөДПЦПуОӘ?Ј»·ҙУҰ·ҪіМКҪОӘ?ЎЈ
ЈЁ4Ј©РҙіцGЦР·ҙУҰөДАлЧУ·ҪіМКҪ?ЎЈ
ЈЁ5Ј©ИфҪ«Cl2әНSO2өИОпЦКөДБҝ»мәПЖҜ°ЧЧчУГјхИхЈ¬УГ»ҜС§·ҪіМКҪҪвКНФӯТт?ЎЈ
ІОҝјҙр°ёЈәЈЁ1Ј©MnO2+4HClЈЁЕЁЈ© MnCl2+Cl2Ўь+2H2O
MnCl2+Cl2Ўь+2H2O
ЈЁ2Ј©ұҘәНNa ClИЬТәЈ»ЕЁ H2SO4?Ј»NaOHИЬТә
ЈЁ3Ј©ҫзБТ·ҙУҰЈ¬ІъЙъЧШәЪЙ«ЈЁЧШәЦЙ«Ј©өДСМЈ¬·ЕіцҙуБҝИИЈ»2 Fe +3 Cl2ЁTЁT2FeCl3 ЈЁМх
јюВФЈ©
ЈЁ4Ј©Cl2+2 OHЈӯЁTЁTClЈӯ+ ClOЈӯ+H2O?(2·Ц)
ЈЁ5Ј©Cl2+SO 2+2H2O ЁTЁTH2SO4+ 2HCl?(2·Ц) ЖдУаёч1·Ц
ұҫМвҪвОцЈәЈЁ1Ј©КөСйКТУГ¶юСх»ҜГМУлЕЁСОЛбјУИИАҙЦЖИЎВИЖшЈ¬№К·ҙУҰОӘMnO2+4HClЈЁЕЁЈ© MnCl2+Cl2Ўь+2H2OЎЈ
MnCl2+Cl2Ўь+2H2OЎЈ
ЈЁ2Ј©ёщҫЭЧ°ЦГҝЙЦӘCЎўDОӘҫ»»ҜәНёЙФпВИЖшөДЧ°ЦГЈ¬ВИЖшТтОӘ»мәП»У·ўіцөДHClЖшМеЈ¬ҝЙУГұҘәНКіСОЛ®іэИҘHClЖшМеЈ¬ВИЖшКЗЛбРФЖшМеҝЙУГЕЁБтЛбёЙФпЎЈёщҫЭЖшМеөДЦЖИЎТӘЗуЎ°ПИҫ»»ҜәуёЙФпЎұ№КCЧ°өДКФјБОӘұҘәНКіСОЛ®Ј¬DЧ°өДКФјБОӘЕЁБтЛбЎЈЧоәуВИЖшөДОьКХУҰёГУГјоТәЗвСх»ҜДЖИЬТәЎЈЛщТФGУҰёГЧ°ЗвСх»ҜДЖИЬТәЎЈ
ЈЁ3Ј©E·ўЙъөДКЗМъУлВИЖш·ҙУҰЙъіЙВИ»ҜМъЎЈПЦПуКЗЈәҫзБТ·ҙУҰЈ¬ІъЙъЧШәЪЙ«ЈЁЧШәЦЙ«Ј©өДСМЈ¬·ЕіцҙуБҝИИЈ»·ҙУҰ·ҪіМКҪОӘ2 Fe +3 Cl2 2FeCl3 ЎЈ
2FeCl3 ЎЈ
ЈЁ4Ј©GЦР·ҙУҰОӘВИЖшУлЗвСх»ҜДЖ·ҙУҰЙъіЙВИ»ҜДЖЎўҙОВИЛбДЖәНЛ®Ј¬АлЧУ·ҪіМКҪОӘЈә
Cl2+2 OHЈӯЁTЁTClЈӯ+ ClOЈӯ+H2O
ЈЁ5Ј©Cl2ҫЯУРЗҝСх»ҜРФәНSO2ҫЯУР»№ФӯРФөИОпЦКөДБҝ»мәПәу·ўЙъСх»Ҝ»№Фӯ·ҙУҰЈә
Cl2+SO 2+2H2O ЁTЁTH2SO4+ 2HCl
өгЖАЈәХЖОХВИЖшөДЦЖИЎФӯБПЎўФӯАнЎўКХјҜЎўёЙФпЎўҫ»»ҜЈ¬ОІЖшҙҰАнөИөИЎЈ
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ДіМъөДСх»ҜОпЈ¬УГ7molЎӨLЎӘ1өДСОЛб100mLФЪТ»¶ЁМхјюПВЗЎәГНкИ«ИЬҪвЈ¬ЛщөГИЬТәФЩНЁИл0.56LұкЧјЧҙҝцПВөДВИЖшКұЈ¬ёХәГК№ИЬТәЦРөДFe2+НкИ«ЧӘ»ҜОӘFe3+ЎЈФтёГСх»ҜОпөД»ҜС§КҪҝЙұнКҫОӘЈЁ?Ј©
AЈ®FeO
BЈ®Fe2O3
CЈ®Fe4O5
DЈ®Fe5O7
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ