1ЎўСЎФсМв јёЦЦ¶МЦЬЖЪФӘЛШөДФӯЧУ°лҫ¶ј°ДіР©»ҜәПјЫјыПВұнЈ¬ПВБРЛө·ЁХэИ·өДКЗЈЁ?Ј©
ФӘЛШҙъәЕ
| A
| B
| D
| E
| G
| H
| I
| J
|
»ҜәПјЫ
| ЁC1
| ЁC2
| +4ЎўЁC4
| +7ЎўЁC1
| +5ЎўЁC3
| +3
| +2
| +1
|
ФӯЧУ°лҫ¶ЈҜnm
| 0Ј®071
| 0Ј®074
| 0Ј®077
| 0Ј®099
| 0Ј®110
| 0Ј®143
| 0Ј®160
| 0Ј®186
|
AЈ®AөДөҘЦКДЬҪ«EөҘЦКҙУHE3өДИЬТәЦРЦГ»»іцАҙ
BЈ®AЎўHЎўJөДАлЧУ°лҫ¶УЙҙуөҪРЎЛіРтКЗAЈҫJЈҫH
CЈ®GФӘЛШөДөҘЦКІ»ҙжФЪН¬ЛШТмРОМе
DЈ®IФЪDB2ЦРИјЙХЙъіЙБҪЦЦ»ҜәПОп
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәёщҫЭФӘЛШөДЧоёЯХэјЫ=ЧоНвІгөзЧУКэЈ¬ЧоёЯХэјЫ+|ЧоөНёәјЫ|=8Ј¬ЧоөНёәјЫ=ЧоНвІгөзЧУКэ-8Ј¬Н¬ЦЬЖЪФӘЛШФӯЧУҙУЧуөҪУТФӯЧУ°лҫ¶ЦрҪҘјхРЎЈ¬Н¬ЦчЧеФӘЛШФӯЧУ°лҫ¶ҙУЙПөҪПВЦрҪҘФцҙуЈ¬ҝЙНЖЦӘЈәAОӘFЈ¬BОӘOЈ¬DОӘCЎўEОӘClЈ¬GОӘPЈ»HОӘAlЈ¬IОӘBeЈ¬JОӘNaЎЈAЎўF2ј«ТЧәНЛ®·ҙУҰЦГ»»іцСхЖшЈ¬ФтAөДөҘЦКІ»ДЬҪ«EөҘЦКҙУHE3өДИЬТәЦРЦГ»»іцАҙЈ¬ҙнОуЈ»BЎўAЎўJЎўH·ЦұрОӘFЎўNaЎўAlЈ¬ЛьГЗөДАлЧУәЛНвөзЧУІгКэПаөИЈ¬АлЧУ°лҫ¶ҙуРЎЛіРтОӘЈәF-ЈҫNa+ЈҫAl3+Ј¬ХэИ·Ј»CЎўәмБЧәН°ЧБЧ»ҘОӘН¬ЛШТмРОМеЈ¬ҙнОуЈ»DЎўҪрКфоләНГҫРФЦКПаК¶Ј¬ФЪ¶юСх»ҜМјЦРөДИјЙХЙъіЙСх»ҜоләНМјөҘЦКЈ¬№КDҙнОуЎЈ
ұҫМвДС¶ИЈәТ»°г
2ЎўМоҝХМв ФЪЛ®ИЬТәЦРЈ¬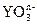 әНS2-·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪИзПВЈә
әНS2-·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪИзПВЈә
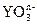 +3S2-+6H+
+3S2-+6H+  ?Y-+3SЎэ+3H2O
?Y-+3SЎэ+3H2O
ЈЁ1Ј©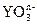 ЦРYФӘЛШөД»ҜәПјЫКЗ?ЎЈ
ЦРYФӘЛШөД»ҜәПјЫКЗ?ЎЈ
ЈЁ2Ј©YФӘЛШФӯЧУөДЧоНвІгөзЧУКэКЗ?ЎЈ
ЈЁ3Ј©ұИҪПS2-әНY-өД»№ФӯРФ?ЎЈ
ІОҝјҙр°ёЈәЈЁ1Ј©+5?ЈЁ2Ј©7?ЈЁ3Ј©S2->Y-
ұҫМвҪвОцЈәУЙ·ҙУҰ·ҪіМКҪЦРөДөзәЙКШәг№ШПөЈ¬өГіцn=1,ЛщТФ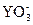 ЦРYФӘЛШОӘ+5јЫЈ¬ө«І»ДЬИ·¶ЁЖдОӘЧоёЯХэјЫЎЈТтYЧоөНёәјЫОӘ-1јЫЈ¬ЛщТФЧоёЯХэјЫОӘ+7јЫЈ¬ЛщТФYЧоНвІгөзЧУКэОӘ7Ј¬ФЪЙПКц·ҙУҰЦРЈ¬S2-ОӘ»№ФӯјБЈ¬Y-ОӘ»№ФӯІъОпЈ¬»№ФӯјБөД»№ФӯРФҙуУЪ»№ФӯІъОпөД»№ФӯРФЈ¬ЛщТФS2-өД»№ФӯРФҙуУЪY-өД»№ФӯРФЎЈ
ЦРYФӘЛШОӘ+5јЫЈ¬ө«І»ДЬИ·¶ЁЖдОӘЧоёЯХэјЫЎЈТтYЧоөНёәјЫОӘ-1јЫЈ¬ЛщТФЧоёЯХэјЫОӘ+7јЫЈ¬ЛщТФYЧоНвІгөзЧУКэОӘ7Ј¬ФЪЙПКц·ҙУҰЦРЈ¬S2-ОӘ»№ФӯјБЈ¬Y-ОӘ»№ФӯІъОпЈ¬»№ФӯјБөД»№ФӯРФҙуУЪ»№ФӯІъОпөД»№ФӯРФЈ¬ЛщТФS2-өД»№ФӯРФҙуУЪY-өД»№ФӯРФЎЈ
ұҫМвДС¶ИЈәјтөҘ
3ЎўСЎФсМв AЎўBЎўC¶јКЗ¶МЦЬЖЪФӘЛШЈ¬ФӯЧУРтКэТАҙОФцҙуЎЈBКЗөШҝЗЦРә¬БҝЧо¶аөДФӘЛШЈ»»ҜәПОпAxByУР¶аЦЦРОКҪЈ¬УРөДҝЙТФөјЦВЛбУкЈ»»ҜәПОпCmBnТІУРІ»Н¬РОКҪЈ¬ЖдЦРmЎўnҝЙТФКЗ2Јә1»т1Јә1ЎЈПВБРЕР¶ПХэИ·өДКЗЈә?
AЎўФӯЧУ°лҫ¶УЙҙуөҪРЎөДЛіРтКЗЈәC>B>A
BЎўAxByЦРAөД»ҜәПјЫІ»ҝЙДЬОӘ+1јЫ
CЎўCУлBРОіЙөД»ҜәПОпИЬУЪЛ®¶јДЬөГөҪөҘЦКB
DЎўAЎўBБҪФӘЛШөДЖшМ¬Зв»ҜОпҝЙТФПа»Ҙ·ҙУҰ
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв
ўЩЛьГЗөДЧоёЯјЫСх»ҜОп¶ФУҰЛ®»ҜОпөДЛбРФBұИAЗҝ
ўЪAөДЖшМ¬Зв»ҜОпұИBөДЖшМ¬Зв»ҜОпОИ¶Ё
ўЫAөДЧоНвІгөзЧУКэұИB¶а
ўЬAәНBФӘЛШөДөҘЦКҫщОӘЛ«ФӯЧУ·ЦЧУ
AЈ®ўЩўЪўЬ
BЈ®ўЩўЬ
CЈ®ўЩўЫ
DЈ®Ц»УРўЩ
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәAЎўBН¬Т»ЦЬЖЪЈ¬ЗТAөДФӯЧУ°лҫ¶ұИBҙуЈ¬ҝЙНЖЦӘAөДФӯЧУРтКэРЎУЪBЈ¬ФтAөДЧеРтКэТІРЎУЪBЈ¬ЗТAөД·ЗҪрКфРФИхУЪBЎЈУЙҙЛЈ¬ҝЙЕР¶ПўЩХэИ·Ј¬ўЪўЫҙнЎЈ¶ФУЪўЬЈ¬УЙУЪМвЦРІ»ДЬГчИ·AЎўBФЪФӘЛШЦЬЖЪұнЦРөДҫЯМеО»ЦГЈ¬ЛщТФЈ¬ОЮ·ЁЕР¶ЁЈ¬·ЦОцёчСЎПоЈ¬Ц»УРDХэИ·ЎЈ
ұҫМвДС¶ИЈәјтөҘ
5ЎўМоҝХМв ЈЁөЪТ»ёцҝХ1·ЦЈ¬ЖдЛьГҝҝХ2·ЦЈ¬№І9·ЦЈ©УРAЎўBЎўCЎўDЎўEОеЦЦ¶МЦЬЖЪФӘЛШЈ¬ТСЦӘПаБЪөДAЎўBЎўCЎўDЛДЦЦФӘЛШФӯЧУәЛНв№ІУР56ёцөзЧУЈ¬ФЪЦЬЖЪұнЦРөДО»ЦГИзНјЛщКҫЎЈEөДөҘЦКҝЙУлЛб·ҙУҰЈ¬1molEөҘЦКУлЧгБҝЛбЧчУГЈ¬ФЪұкЧјЧҙҝцПВДЬІъЙъ33.6LH2Ј»EөДСфАлЧУУлAөДТхАлЧУәЛНвөзЧУІгҪб№№НкИ«ПаН¬ЎЈ
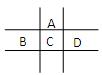
»ШҙрПВБРОКМвЈә
ЈЁ1Ј©BөДФӘЛШГыіЖОӘ??Ј¬DФЪЦЬЖЪұнЦРөДО»ЦГ?ЎЈ
ЈЁ2Ј©A,C,D¶ФУҰөДАлЧУ°лҫ¶УЙҙуөҪРЎЛіРтОӘ?Ј¬ЈЁУГ»ҜС§·ыәЕұнКҫЈ©
ЈЁ3Ј©DөДөҘЦКУлЛ®·ҙУҰөДАлЧУ·ҪіМКҪОӘ_?ЎЈ
ЈЁ4Ј©ПтDУлEРОіЙөД»ҜәПОпөДЛ®ИЬТәЦРөОИл№эБҝЙХјоИЬТәЈ¬УГАлЧУ·ҪіМКҪұнКц?
?ЎЈ
ІОҝјҙр°ёЈәЈЁ1Ј©БЧЈ¬?өЪИэЦЬЖЪўчAЧе
ЈЁ2Ј© S2ЎӘ>ClЎӘ>O2-
ЈЁ3Ј©Cl2Ј«H2OЈҪH++ClЎӘЈ«HClO
ЈЁ4Ј©Al3Ј«Ј«4OHЈӯЈӯЈҪAlO2ЈӯЈ«2H2O
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г