1ЎўСЎФсМв БҪ·ЭВБРјЈ¬өЪТ»·ЭУлСОЛб·ҙУҰЈ¬өЪ¶ю·ЭУлNaOHИЬТә·ҙУҰЈ¬ІъЙъөДЗвЖшөДМе»эұИОӘ1©U2Ј¬БҪ·ЭВБРјЦРІОјУ·ҙУҰөДВБөДЦКБҝұИОӘЈЁ Ј©
AЈ®1©U1
BЈ®1©U2
CЈ®1©U3
DЈ®2©U1
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв іЖИЎБҪ·ЭВБ·ЫЈ¬өЪТ»·ЭјУИлЧгБҝөДЕЁЗвСх»ҜДЖИЬТәЈ¬өЪ¶ю·ЭјУИлЧгБҝөДПЎСОЛбЈ¬Из№ыТӘ·ЕіцПаН¬өДЖшМеЈЁФЪН¬ОВН¬С№ПВЈ©Ј¬БҪ·ЭВБөДЦКБҝЦ®ұИОӘЈә
AЈ®1©U2
BЈ®1©U3
CЈ®3©U2
DЈ®1©U1
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә2Al+2NaOH+6H2O="2" Na[Al(OH)4]+3H2Ўь? 2Al+6HCl=2AlCl3+3H2Ўь,№КDХэИ·
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв Птә¬2mol KAl(SO4)2өДИЬТәЦРјУИл2L 3.5mol/LNaOHИЬТәЈ¬ФтВБФӘЛШөДҙжФЪРОКҪУР
AЈ®И«ІҝОӘAlO2ЎӘ
BЈ®И«ІҝОӘAl(OH)3
CЈ®Al(OH)3әНAlO2ЎӘ
DЈ®Al(OH)3әНAl3+
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈә2molAl3+Ј¬әН6molNaOH·ҙУҰЙъіЙAl(OH)3,әН8molNaOHЙъіЙNaAlO2Ј¬јУИл7molNaOHЈ¬№Кҙр°ёОӘCЎЈ
ұҫМвДС¶ИЈәјтөҘ
4ЎўСЎФсМв ФЪ1Lә¬0.1 mol NaAlO2әН0.1 mol Ba(OH)2өД»мәПИЬТәЦРЈ¬ЦрөОјУИлЕЁ¶ИОӘ0.1 molЎӨL-1өДH2SO4ИЬТәЈ¬ЛщІъЙъөДіБөнөДОпЦКөДБҝәНЛщјУИлБтЛбИЬТәөДМе»э№ШПөҝЙУГПВБРНјПсұнКҫөДКЗЈЁ?Ј©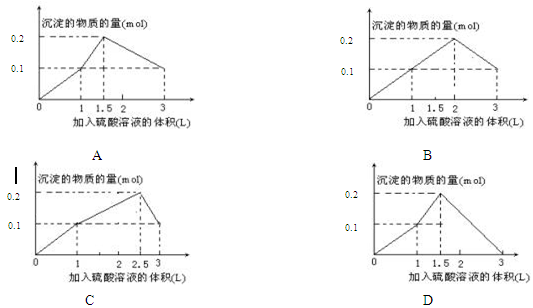
ІОҝјҙр°ёЈәA
ұҫМвҪвОцЈәПт0.1 mol NaAlO2әН0.1 mol Ba(OH)2өД»мәПИЬТәЦРЈ¬ЦрөОјУИл0.1 molЎӨL-1өДH2SO4ИЬТәЈ¬ПИУлBa(OH)2·ҙУҰЈ¬РиПыәДБтЛбөДОпЦКөДБҝОӘ0.1 molЈ¬јҙПыәДБтЛбИЬТәөДМе»эОӘ1LЈ¬ФЪХвёц№эіМЦРЈ¬ЗЎәГНкИ«·ҙУҰЙъіЙBaSO4әНH2OЈ¬ФЩЦрөОөОИлH2SO4УлNaAlO2·ҙУҰЈ¬ЖдАлЧУ·ҪіМКҪОӘH++ALO2ЎӘ+H2O=AL(OH)3ЎэЈ»өұјУИлБтЛбөДОпЦКөДБҝҙпөҪ0.05molКұЈ¬ALO2ЎӘИ«ІҝЧӘ»ҜОӘAL(OH)3ЎэЈ»ФЩјУИлБтЛбЈ¬AL(OH)3ЦрҪҘИЬҪвЈ¬Жд·ҙУҰАлЧУ·ҪіМКҪОӘAL(OH)3+3H+=AL3++3H2OЈ»өұјУИлБтЛбөДОпЦКөДБҝҙпөҪ0.15molКұЈ¬AL(OH)3И«ІҝИЬҪвЈ¬ФЩјУИлБтЛбЈ¬іБөнЦКБҝІ»ұдЎЈ
ЧЫЙПЛщКцЈ¬ХэИ·СЎПоОӘAЈ»
ұҫМвДС¶ИЈәТ»°г
5ЎўМоҝХМв AЎўBЎўXЎўYҫщОӘЦРС§ҪЧ¶ОөДіЈјыОпЦКЈ¬ЛьГЗЦ®јдөДЧӘ»Ҝ№ШПөИзНјЛщКҫЈә
Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ИфAОӘҪрКфөҘЦКЈ¬BОӘ·ЗҪрКфСх»ҜОпЈ¬ФтёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘ______Ј»ЗлРҙіцЦКБҝКэОӘ26өДAөДТ»ЦЦәЛЛШ·ыәЕ______Ј®
ЈЁ2Ј©ИфAОӘҪрКфөҘЦКЈ¬BОӘәЪЙ«ҙЕРФҫ§МеЈ¬ФтёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘ______Ј»ЗлРҙіцAУлЗвСх»ҜДЖИЬТә·ҙУҰөДАлЧУ·ҪіМКҪ______Ј®
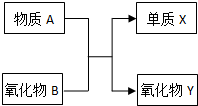
ІОҝјҙр°ёЈәЈЁ1Ј©ИфAОӘҪрКфөҘЦКЈ¬BОӘ·ЗҪрКфСх»ҜОпЈ¬ёГ·ҙУҰОӘЦГ»»·ҙУҰЈ¬·ыәПМхјюөДУРЈәМъәНЛ®ХфЖшөД·ҙУҰЎўГҫәН¶юСх»ҜМј·ўЙъөДЦГ»»·ҙУҰЈ¬Из№ыAКЗГҫЈ¬BКЗ¶юСх»ҜМјЈ¬ФтёГ·ҙУҰ·ҪіМКҪОӘЈә2Mg+CO2өгИј.2MgO+CЈ¬
Из№ыAөДЦКБҝКэОӘ26Ј¬ФтAКЗГҫФӘЛШЈ¬ЖдЦКЧУКэКЗ12Ј¬ФтЖдәЛЛШ·ыәЕОӘ2612MgЈ¬
№Кҙр°ёОӘЈә2Mg+CO2өгИј.2MgO+CЈ»2612MgЈ»
ЈЁ2Ј©ИфAОӘҪрКфөҘЦКЈ¬BОӘәЪЙ«ҙЕРФҫ§МеЈ¬ёГ·ҙУҰОӘВБИИ·ҙУҰЈ¬ФтXКЗМъЈ¬YКЗСх»ҜВБЈ¬ёГ·ҙУҰ·ҪіМКҪОӘЈә8Al+3Fe3O4өгИј.9Fe+4Al2O3Ј¬ВБәНЗвСх»ҜДЖИЬТә·ҙУҰЙъіЙЖ«ВБЛбДЖәНЗвЖшЈ¬ёГ·ҙУҰАлЧУ·ҪіМКҪОӘЈә2Al+2OH-+2H2O=2AlO2-+3H2ЎьЈ¬
№Кҙр°ёОӘЈә8Al+3Fe3O4өгИј.9Fe+4Al2O3Ј»2Al+2OH-+2H2O=2AlO2-+3H2ЎьЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ