1ЎўСЎФсМв БҪ·ЭВБРјЈ¬өЪТ»·ЭУлСОЛб·ҙУҰЈ¬өЪ¶ю·ЭУлNaOHИЬТә·ҙУҰЈ¬ІъЙъөДЗвЖшөДМе»эұИОӘ1©U2Ј¬БҪ·ЭВБРјЦРІОјУ·ҙУҰөДВБөДЦКБҝұИОӘЈЁ Ј©
AЈ®1©U1
BЈ®1©U2
CЈ®1©U3
DЈ®2©U1
2ЎўСЎФсМв іЖИЎБҪ·ЭВБ·ЫЈ¬өЪТ»·ЭјУИлЧгБҝөДЕЁЗвСх»ҜДЖИЬТәЈ¬өЪ¶ю·ЭјУИлЧгБҝөДПЎСОЛбЈ¬Из№ыТӘ·ЕіцПаН¬өДЖшМеЈЁФЪН¬ОВН¬С№ПВЈ©Ј¬БҪ·ЭВБөДЦКБҝЦ®ұИОӘЈә
AЈ®1©U2
BЈ®1©U3
CЈ®3©U2
DЈ®1©U1
3ЎўСЎФсМв Птә¬2mol KAl(SO4)2өДИЬТәЦРјУИл2L 3.5mol/LNaOHИЬТәЈ¬ФтВБФӘЛШөДҙжФЪРОКҪУР
AЈ®И«ІҝОӘAlO2ЎӘ
BЈ®И«ІҝОӘAl(OH)3
CЈ®Al(OH)3әНAlO2ЎӘ
DЈ®Al(OH)3әНAl3+
4ЎўСЎФсМв ФЪ1Lә¬0.1 mol NaAlO2әН0.1 mol Ba(OH)2өД»мәПИЬТәЦРЈ¬ЦрөОјУИлЕЁ¶ИОӘ0.1 molЎӨL-1өДH2SO4ИЬТәЈ¬ЛщІъЙъөДіБөнөДОпЦКөДБҝәНЛщјУИлБтЛбИЬТәөДМе»э№ШПөҝЙУГПВБРНјПсұнКҫөДКЗЈЁ?Ј©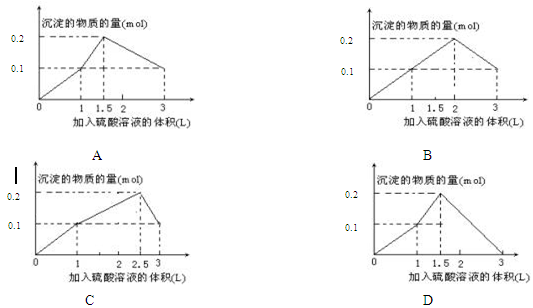
5ЎўМоҝХМв AЎўBЎўXЎўYҫщОӘЦРС§ҪЧ¶ОөДіЈјыОпЦКЈ¬ЛьГЗЦ®јдөДЧӘ»Ҝ№ШПөИзНјЛщКҫЈә
Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ИфAОӘҪрКфөҘЦКЈ¬BОӘ·ЗҪрКфСх»ҜОпЈ¬ФтёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘ______Ј»ЗлРҙіцЦКБҝКэОӘ26өДAөДТ»ЦЦәЛЛШ·ыәЕ______Ј®
ЈЁ2Ј©ИфAОӘҪрКфөҘЦКЈ¬BОӘәЪЙ«ҙЕРФҫ§МеЈ¬ФтёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘ______Ј»ЗлРҙіцAУлЗвСх»ҜДЖИЬТә·ҙУҰөДАлЧУ·ҪіМКҪ______Ј®