1ЎўСЎФсМв ТСЦӘ·ҙУҰC+O2ЁTCO2ОӘ·ЕИИ·ҙУҰЈ¬¶ФёГ·ҙУҰөДПВБРЛө·ЁХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®CөДДЬБҝТ»¶ЁёЯУЪCO2
BЈ®O2өДДЬБҝТ»¶ЁёЯУЪCO2
CЈ®CәНO2өДДЬБҝТ»¶ЁёЯУЪCO2өДДЬБҝ
DЈ®CәНO2өДДЬБҝТ»¶ЁөНУЪCO2өДДЬБҝ
2ЎўСЎФсМв ПВБРУР№ШДЬБҝЧӘ»ҜөДАнҪвІ»ХэИ·өДКЗ
[? ]
AЈ®ПЦФЪК№УГөДёчПоХХГчЙиұёКЗҪ«өзДЬЧӘ»ҜОӘ№вДЬ
BЈ®ИјБПөДИјЙХ№эіМЦ»КЗҪ«ФМІШөД»ҜС§ДЬЧӘ»ҜОӘИИДЬ
CЈ®·зДЬКЗМ«СфДЬөДТ»ЦЦЧӘ»»РОКҪЈ¬·зДЬҝЙТФФЩЧӘ»ҜОӘөзДЬ
DЈ®ЙъОпМеДЪөДЙъОп»ҜС§№эіМөДДЬБҝЧӘ»ҜФЪЎ°ДЬФҙЎұАыУГЙПёьОӘәПАнЎўУРР§
3ЎўМоҝХМв (10·ЦЈ©ТСЦӘФЪ250CЎў1.013ЎБ105PaПВЈ¬1molЗвЖшНкИ«ИјЙХЙъіЙТәМ¬Л®·Еіц285kJөДИИБҝЈ¬Зл»ШҙрПВБРОКМвЈәЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ
ЈЁ1Ј©Иф2 molЗвЖшНкИ«ИјЙХЙъіЙЛ®ХфЖшЈ¬Фт·ЕіцөДИИБҝ ___ЈЁМоЎ°ЈҫЎұЎўЎ°ЈјЎұ»тЎ°ЈҪЎұЈ©570 kJ
ЈЁ2Ј©ПЦУР250CЎў1.013ЎБ105PaПВөДH2әНCH4өД»мәПЖшМе0.5molЈ¬НкИ«ИјЙХЙъіЙТ»¶ЁЦКБҝ
өДCO2ЖшМеәН10.8gH2OЈЁlЈ©Ј¬·Еіц203kJөДИИБҝЈ¬Фт1molCH4?НкИ«ИјЙХЙъіЙCO2
ЖшМеәНH2OЈЁlЈ©өДИИ»ҜС§·ҪіМКҪОӘ ____________________________________ ЎЈ
ЈЁ3Ј©ГА№ъ°ўІЁВЮУоЦж·Йҙ¬ЙПК№УГБЛТ»ЦЦРВРНЧ°ЦГЈ¬Жд№№ФмИзНјЛщКҫЈәAЎўBБҪёцөзј«
ҫщУЙ¶аҝЧөДМјҝйЧйіЙЎЈ

ёГөзіШөДХэј«ОӘЈә__________ЈЁМоA»тBЈ©ЎЈИфёГөзіШ№ӨЧчКұФцјУБЛ1mol H2OЈ¬өз
В·ЦРЧӘТЖөзЧУөДОпЦКөДБҝОӘ ____________ ЎЈ
ЈЁ4Ј©Из№ыҪ«ЙПКцЧ°ЦГЦРНЁНщөДH2ёДіЙCH4ЖшМеЈ¬ТІҝЙТФЧйіЙТ»ёцФӯөзіШЧ°ЦГЈ¬өзіШөДЧЬ·ҙУҰ·ҪіМКҪОӘЈәCH4+2O2+2KKOH=K2CO3+3H2OЈ¬ФтёГөзіШөДёәј«·ҙУҰКҪОӘЈә
______________________________________ЎЈ
4ЎўјтҙрМв ДіКР¶ФҙуЖшҪшРРјаІв,·ўПЦёГКРКЧТӘОЫИҫОпОӘҝЙОьИлҝЕБЈОпPM2.5(Цұҫ¶РЎУЪөИУЪ2.5ҰМmөДРьёЎҝЕБЈОп),ЖдЦчТӘАҙФҙОӘИјГәЎў»ъ¶ҜіөОІЖшөИЎЈТтҙЛ,¶ФPM2.5ЎўSO2ЎўNOxөИҪшРРСРҫҝҫЯУРЦШТӘТвТеЎЈ
Зл»ШҙрПВБРОКМв:
(1)Ҫ«PM2.5СщұҫУГХфБуЛ®ҙҰАнЦЖіЙҙэІвКФСщЎЈ
ИфІвөГёГКФСщЛщә¬Л®ИЬРФОЮ»ъАлЧУөД»ҜС§Чй·Цј°ЖдЖҪҫщЕЁ¶ИИзПВұн:
АлЧУ
| K+
| Na+
| N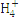
| S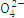
| N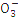
| Cl-
|
ЕЁ¶И/molЎӨL-1
| 4ЎБ10-6
| 6ЎБ10-6
| 2ЎБ10-5
| 4ЎБ10-5
| 3ЎБ10-5
| 2ЎБ10-5
|
ёщҫЭұнЦРКэҫЭЕР¶ПPM2.5өДЛбјоРФОӘЎЎЎЎЎЎЎЎЎЎЎЎ,КФСщөДpH=ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ
(2)ОӘјхЙЩSO2өДЕЕ·Е,іЈІЙИЎөДҙлК©УР:
ўЩҪ«ГәЧӘ»ҜОӘЗеҪаЖшМеИјБПЎЈ
ТСЦӘ:H2(g)+ O2(g) H2O(g)?ҰӨH="-241.8" kJЎӨmol-1?ўЩ
O2(g) H2O(g)?ҰӨH="-241.8" kJЎӨmol-1?ўЩ
C(s)+ O2(g) CO(g)ЎЎ?ҰӨH="-110.5" kJЎӨmol-1?ўЪ
O2(g) CO(g)ЎЎ?ҰӨH="-110.5" kJЎӨmol-1?ўЪ
РҙіцҪ№МҝУлЛ®ХфЖш·ҙУҰөДИИ»ҜС§·ҪіМКҪ:ЎЎ?ЎЈ
ўЪПҙөУә¬SO2өДСМЖшЎЈТФПВОпЦКҝЙЧчПҙөУјБөДКЗЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЈ
a.Ca(OH)2ЎЎЎЎ? b.Na2CO3ЎЎ?ЎЎc.CaCl2ЎЎ?ЎЎd.NaHSO3
(3)ЖыіөОІЖшЦРNOxәНCOөДЙъіЙј°ЧӘ»Ҝ
ўЩТСЦӘЖыёЧЦРЙъіЙNOөД·ҙУҰОӘ:
N2(g)+O2(g) 2NO(g)ЎЎҰӨH>0
2NO(g)ЎЎҰӨH>0
Иф1 molҝХЖшә¬0.8 mol N2әН0.2 mol O2,1 300ЎжКұФЪГЬұХИЭЖчДЪ·ҙУҰҙпөҪЖҪәв,ІвөГNOОӘ8ЎБ10-4molЎЈјЖЛгёГОВ¶ИПВөДЖҪәвіЈКэK=ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ?ЎЈ
ЖыіөЖф¶Ҝәу,ЖыёЧОВ¶ИФҪёЯ,өҘО»КұјдДЪNOЕЕ·ЕБҝФҪҙу,ФӯТтКЗЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ?ЎЎЎЎЎЈ
ўЪЖыіөИјУНІ»НкИ«ИјЙХКұІъЙъCO,УРИЛЙиПл°ҙПВБР·ҙУҰіэИҘCO:
2CO(g) 2C(s)+O2(g)
ТСЦӘёГ·ҙУҰөДҰӨH>0,јтКцёГЙиПлДЬ·сКөПЦөДТАҫЭ: ?
?ЎЈ
ўЫДҝЗ°,ФЪЖыіөОІЖшПөНіЦРЧ°ЦГҙЯ»ҜЧӘ»ҜЖчҝЙјхЙЩCOәНNOөДОЫИҫ,Жд»ҜС§·ҙУҰ·ҪіМКҪОӘЎЎ?ЎЈ
5ЎўСЎФсМв ЈЁ1Ј©ТСЦӘЈәФЪ1ЎБ105PaМхјюПВЈ¬ЗвЖшөДұкЧјИјЙХИИКЗ285.8 kJЎӨmolЈӯ1,ПВБРИИ»ҜС§·ҪіМКҪХэИ·өДКЗ ЈЁ?Ј©
AЈ®H2OЈЁgЈ©ЈҪH2ЈЁgЈ©Ј«1/2O2ЈЁgЈ©ҰӨHЈҪЈ«285.8kJЎӨmolЈӯ1
BЈ®2H2ЈЁgЈ©Ј«O2ЈЁgЈ©ЈҪ2H2OЈЁlЈ©ҰӨHЈҪЎӘ517.6kJЎӨmolЈӯ1
CЈ®H2ЈЁgЈ©Ј«1/2 O2ЈЁgЈ©ЈҪH2OЈЁgЈ©ҰӨHЈҪЎӘ285.8kJЎӨmolЈӯ1-
DЈ®2H2ЈЁgЈ©Ј«O2ЈЁgЈ©ЈҪ2H2OЈЁlЈ©ҰӨHЈҪЈ«517.6JЎӨmolЈӯ1