1ЎўСЎФсМв ПВБРАлЧУ·ҪіМКҪКйРҙХэИ·өДКЗ
[? ]
AЈ®ЗвСх»ҜВБИЬУЪЗвСх»ҜДЖИЬТәЈәAl (OH)3+3OH- = AlO2- + 3H2O
BЈ®AlCl3ИЬТәЦРјУИлЧгБҝөД°ұЛ®ЈәAl3++ 3OH- ЁT Al(OH)3Ўэ
CЈ®УГFeCl3ИЬТәёҜКҙНӯөзВ·°еЈә2Fe3++ Cu = 2Fe2++ Cu2+
DЈ®НӯЖ¬јУИлЕЁПхЛбЦРЈәCu+4H++2NO3-=Cu2++2H2O+2NO2Ўь
ІОҝјҙр°ёЈәCD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪХэИ·өДКЗ
AЈ®Бт»ҜСЗМъЦРјУИлПЎБтЛб 2H++S2ЈӯЎъH2SЎь


BЈ®ПтұҪ·УДЖИЬТәЦРНЁИлЙЩБҝөДCO2ЈӯOЈӯЈ«CO2Ј«H2OЎъЈӯOHЈ«HCO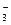
CЈ®ПтМјЛбЗвДЖИЬТәЦРөОИлЙЩБҝКҜ»ТЛ® HCO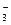 +Ca2++OHЈӯЎъCaCO3Ўэ+H2O
+Ca2++OHЈӯЎъCaCO3Ўэ+H2O
DЈ®ПтГч·ҜИЬТәЦРјУИл№эБҝөД°ұЛ® A13++3NH3ЎӨH2OЎъAl(OH)3Ўэ+3NH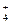
ІОҝјҙр°ёЈәBD
ұҫМвҪвОцЈәВФ
ұҫМвДС¶ИЈәјтөҘ
3ЎўСЎФсМв ДЬУГH+?+ OHЈӯ ЈҪH2OАҙұнКҫөД»ҜС§·ҙУҰКЗЈЁ?Ј©
AЈ®ЗвСх»ҜГҫәНПЎСОЛб·ҙУҰ
BЈ®Ba(OH)2ИЬТәөОИлПЎБтЛбЦР
CЈ®БтЛбУл°ұЛ®»мәП
DЈ®іОЗеКҜ»ТЛ®әНПЎПхЛб·ҙУҰ
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈәAЗвСх»ҜГҫДСИЭЈ¬І»ДЬРҙіЙАлЧУРОКҪЈ¬ҙнОуЎЈBөДАлЧУ·ҪіМКҪ2H++SO42++Ba2++2OH-=BaSO4Ўэ+2H2O,ҙнОуЎЈТ»Л®әП°ұКЗИхјоЈ¬І»ДЬРҙіЙАлЧУЈ¬ҙнОуЎЈD КЗЗҝЛбУлЗҝјоЙъіЙИЭТЧИЬҪвЎўТЧөзАлөДСОөД·ҙУҰЈ¬ХэИ·ЎЈ
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв ПВБР·ҙУҰөДАлЧУ·ҪіМКҪХэИ·өДКЗ
[? ]
AЈ®УГЕЁСОЛбЛб»ҜөДKMnO4ИЬТәУлH2O2·ҙУҰЈ¬ЦӨГчH2O2ҫЯУР»№ФӯРФЈә2MnO4-+6H++5H2O2=2Mn2++5O2Ўь
+8H2O
BЈ®ЖҜ°Ч·ЫИЬТәФЪҝХЖшЦРК§Р§ЈәClO-+CO2+H2O=HClO+HCO3-
CЈ®Fe(OH)3ИЬУЪЗвөвЛбЈәFe(OH)3+3H+=Fe3++3H2O
DЈ®Птде»ҜСЗМъИЬТәЦРНЁИлөИОпЦКөДБҝөДВИЖшЈә2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl-
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ПВБРАлЧУ·ҙУҰ·ҪіМКҪХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®іОЗеКҜ»ТЛ®АҙОьКХВИЖшЈәCl2+OH-=Cl-+ClO-+H+
BЈ®Ҫ«ҪрКфNaјУИлАдЛ®ЦРЈә2Na+2H2O=2Na++2OH-+H2Ўь
CЈ®ПтПЎПхЛбЦРјУИлЙЩБҝМъ·ЫЈә3Fe+8H++2NO3-=3Fe2++2NOЎь+4H2O
DЈ®ФЪМјЛбЗвёЖИЬТәЦРјУИлЙЩБҝҝБРФјШИЬТәЈәCa2++2HCO3-+2OH-=CaCO3Ўэ+CO32-+2H2O
ІОҝјҙр°ёЈәAЎўВИЖшәНЗҝјо·ҙУҰөДКөЦККЗЈәCl2+2OH-=Cl-+ClO-+H2OЈ¬№КAҙнОуЈ»
BЎўДЖәНЛ®ҫзБТ·ҙУҰ·ЕЗвЖшН¬КұЙъіЙЗвСх»ҜДЖЈ¬јҙ2Na+2H2O=2Na++2OH-+H2ЎьЈ¬№КBХэИ·Ј»
CЎўПтПЎПхЛбЦРјУИлЙЩБҝМъ·ЫЈәFe+4H++NO3-=Fe3++NOЎь+2H2OЈ¬№КCҙнОуЈ»
DЎўФЪМјЛбЗвёЖИЬТәЦРјУИлЙЩБҝҝБРФјШИЬТәЈ¬·ҙУҰОӘЈәCa2++HCO3-+OH-=CaCO3Ўэ+H2OЈ¬№КDҙнОуЈ®
№КСЎBЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәјтөҘ