| ёЯҝјКЎј¶өјәҪ | |
|
|
| ёЯҝјКЎј¶өјәҪ | |
|
|
|
ёЯҝј»ҜС§ұШҝјЦӘК¶өгЎ¶Л®өДөзАлЖҪәвЎ·Б·П°Мв(¶ю)
ІОҝјҙр°ёЈәD ұҫМвҪвОцЈәФЪИЬТәЦРЈ¬HClөзАлКЗІ»ҝЙДжөДЈ¬¶шCH3COOHөДөзАлКЗҝЙДжөДЈ¬ҙжФЪөзАлЖҪәвЈ¬ФЪјУЛ®ПЎКНөД№эіМЦРЈ¬HClИЬТәАпc(H+)өДЦчТӘұд»ҜЦ»УРТ»ёцЈ¬јҙјхРЎЈ»CH3COOHИЬТәАпc(H+)өДЦчТӘұд»ҜУРБҪёцЈ¬јҙјхРЎәНФцҙуЎЈИфa=bЈ¬ПЎКНәуөДCH3COOHИЬТәpH<5Ј¬ИфК№CH3COOHИЬТәpH=5Ј¬ҫНұШРлјУЛ®ПЎКНЈ¬јҙa>bЎЈ ұҫМвДС¶ИЈәТ»°г 3ЎўСЎФсМв ўЩpH=0өДСОЛб?ўЪ0.5mol?L-1СОЛб?ўЫ0.1mol?L-1өДNH4ClИЬТә?ўЬ0.1mol?L-1өДNaOHИЬТә?ўЭ0.5mol?L-1өДNH4ClИЬТәЈ¬ТФЙПИЬТәЦРЛ®өзАлөДcЈЁH+Ј©УЙҙуөҪРЎөДЛіРтКЗЈЁЎЎЎЎЈ© ІОҝјҙр°ёЈәA ұҫМвҪвОцЈә ұҫМвДС¶ИЈәјтөҘ 4ЎўСЎФсМв іЈОВПВЈ¬¶ФУЪpH = 11өДNaOHИЬТәЈ¬ПВБРЛө·ЁҙнОуөДКЗ |
ІОҝјҙр°ёЈәC
ұҫМвҪвОцЈәAЈ®ёщҫЭөзәЙКШәгҝЙөГc(OHЈӯ) =c(Na+) + c(H+)Ј¬ХэИ·Ј»BЈ® pH = 11өДNaOHИЬТә, c(H+)= 1Ј®0ЎБ 10-11mol/L Ј¬УЙУЪФЪКТОВПВЛ®өДАлЧУ»эКЗёціЈКэЈ¬ЛщТФёГИЬТәЦРөДc(OHЈӯ) = 1Ј®0ЎБ10-3 molЎӨL-1Ј¬ХэИ·Ј»CЈ®NaOHКЗЗҝјоЈ¬НкИ«өзАлЈ¬ҙЧЛбКЗИхЛбЈ¬Іҝ·ЦөзАлЈ¬өұУлөИМе»эpH = 3өДҙЧЛб»мәПәуУЙУЪЛб№эБҝЈ¬ЛщТФЛщөГИЬТәПФЛбРФЈ¬ҙнОуЈ»DЈ®УлөИМе»э0Ј®001 molЎӨL-1өДВИ»Ҝ炙мәПәуЛщөГИЬТә°®»ӨДгТФөИЕЁ¶ИөДNaClЎўNH3?H2O,УЙУЪТ»Л®әП°ұКЗИхјоЈ¬Іҝ·ЦөзАлЈ¬ФЪИЬТәЦРҙжФЪөзАлЖҪәвЈ¬ЛщТФc(Na+)>c(NH4+)Ј¬ХэИ·ЎЈ
ҝјөгЈәҝјІйИЬТәЦРАлЧУЕЁ¶ИҙуРЎұИҪПЎўИЬТәЛбјоРФөДЕР¶ПөДЦӘК¶ЎЈ
ұҫМвДС¶ИЈәТ»°г
5ЎўСЎФсМв ПВБРРрКцХэИ·өДКЗ
AЈ®c(NH4+)ПаөИөД(NH4)2SO4Ўў(NH4)2Fe(SO4)2әНNH4ClИЬТәЦРЈ¬ИЬЦКЕЁ¶ИҙуРЎ№ШПөКЗЈә
c[(NH4)2Fe(SO4)2]Јјc[(NH4)2SO4]Јјc(NH4Cl)
BЈ®Иф0.3 mol/L HYИЬТәУл0.3 mol/L NaOHИЬТәөИМе»э»мәПәуЈ¬ИЬТәөДpH=9Ј¬Фт
c(OHЈӯ)Јӯc(HY)=c(H+)=1ЎБ10Јӯ5mol/L
CЈ®0.2molЎӨLЈӯ1 HClИЬТәУлөИМе»э0.05 molЎӨLЈӯ1 Ba(OH)2ИЬТә»мәПәуЈ¬ИЬТәөДpH=1
DЈ®NaHCO3ИЬТәЦРЈәc(H+)+c(H2CO3)=c(CO32Јӯ)+c(OHЈӯ)
ІОҝјҙр°ёЈәAD
ұҫМвҪвОцЈәёщҫЭСОөДЧйіЙҝЙЦӘЈ¬ИЬЦКЕЁ¶ИПаН¬КұЈ¬БтЛбп§ИЬТәЦРөДп§ёщАлЧУЕЁ¶ИұИВИ»Ҝп§ИЬТәЦРөДҙ󣬶шБтЛбСЗМъп§ИЬТәЦРөДп§ёщАлЧУЕЁ¶ИұИБтЛбп§ЦРРЎЈ¬ТтОӘСЗМъАлЧУөДЛ®Ҫв·ҙУҰДЬТЦЦЖп§ёщАлЧУөДЛ®ҪвЈ¬Фтc(NH4+)Јә(NH4)2Fe(SO4)2>(NH4)2SO4>NH4ClЈ¬·ҙЦ®Ј¬ИфИэЦЦп§СОИЬТәЦРc(NH4+)ПаөИЈ¬БтЛбСЗМъп§ЕЁ¶ИұЈіЦІ»ұдКұЈ¬ФтВИ»Ҝп§ЕЁ¶ИФцҙуөДіМ¶ИЧоҙуЈ¬БтЛбп§ЕЁ¶ИФцҙуөДіМ¶ИЖдҙОЈ¬ЛщТФИЬЦКЕЁ¶ИҙуРЎ№ШПөОӘc[(NH4)2Fe(SO4)2]Јјc[(NH4)2SO4]Јјc(NH4Cl)Ј¬№КAСЎПоХэИ·Ј»УЙУЪn=c?VЈ¬ФтHYәНNaOHөДОпЦКөДБҝПаөИЈ¬¶юХЯЗЎәГНкИ«ЦРәНЈ¬ЙъіЙNaYИЬТәЈ¬УЙУЪИЬТәpH=9Ј¬јҙИЬТәПФјоРФЈ¬ЛөГчNaYКЗЗҝјоИхЛбСОЎўHYКЗИхЛбЈ¬ПаН¬Ј¬УЙУЪc(H+)=10ЎӘpHmol/L=10ЎӘ9mol/LЈ¬Kw=c(H+)?c(OHЈӯ)=10ЎӘ14Ј¬ФтёГјоРФИЬТәЦРөДИЬТәЦРc(OHЈӯ)=Kw/c(H+)=10ЎӘ5mol/LЈ¬УЙУЪNaYИЬТәЦРЦКЧУКШәг№ШПөКҪОӘc(OHЈӯ)=c(H+)+c(HY)= 10ЎӘ5mol/LЈ¬№КBСЎПоҙнОуЈ»ЗҝЛбУлЗҝјо»мәПКұЗҝЛб№эБҝЈ¬Фт»мәПәуИЬТәЦРc(H+)=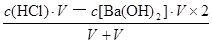 =
=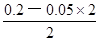 mol/L=0.05mol/LЈ¬УЙУЪpH="ЎӘlg" c(H+)Ј¬ФтёГИЬТәpHҪйУЪ1Ў«2Ц®јдЈ¬№КCСЎПоҙнОуЈ»МјЛбЗвДЖөДөзАліМ¶ИФ¶ҙуУЪЛ®Ј¬јҙNaHCO3=Na++HCO3ЎӘЎўH2O
mol/L=0.05mol/LЈ¬УЙУЪpH="ЎӘlg" c(H+)Ј¬ФтёГИЬТәpHҪйУЪ1Ў«2Ц®јдЈ¬№КCСЎПоҙнОуЈ»МјЛбЗвДЖөДөзАліМ¶ИФ¶ҙуУЪЛ®Ј¬јҙNaHCO3=Na++HCO3ЎӘЎўH2O H++OHЎӘЈ¬Л®өзАліцөДЗвАлЧУЕЁ¶ИөИУЪЛ®өзАліцөДЗвСхёщАлЧУЈ¬МјЛбЗвёщАлЧУІ»ҪцДЬЛ®ҪвЈ¬¶шЗТДЬөзАлЈ¬јҙHCO3ЎӘ+ H2O
H++OHЎӘЈ¬Л®өзАліцөДЗвАлЧУЕЁ¶ИөИУЪЛ®өзАліцөДЗвСхёщАлЧУЈ¬МјЛбЗвёщАлЧУІ»ҪцДЬЛ®ҪвЈ¬¶шЗТДЬөзАлЈ¬јҙHCO3ЎӘ+ H2O H2CO3+OHЎӘЎўHCO3ЎӘ
H2CO3+OHЎӘЎўHCO3ЎӘ H++ CO32ЎӘЈ¬З°ХЯПыәДЛ®өзАліцөДЗвАлЧУЕЁ¶ИУлЙъіЙөДМјЛбЕЁ¶ИПаөИЈ¬әуХЯПыәДЛ®өзАліцөДЗвСхёщАлЧУЕЁ¶ИУлЙъіЙөДМјЛбёщАлЧУЕЁ¶ИПаөИЈ¬ёщҫЭЦКЧУКШәгФӯАнҝЙөГЈәc(H+)+c(H2CO3)=c(CO32Јӯ)+c(OHЈӯ)Ј¬№КDСЎПоХэИ·ЎЈ
H++ CO32ЎӘЈ¬З°ХЯПыәДЛ®өзАліцөДЗвАлЧУЕЁ¶ИУлЙъіЙөДМјЛбЕЁ¶ИПаөИЈ¬әуХЯПыәДЛ®өзАліцөДЗвСхёщАлЧУЕЁ¶ИУлЙъіЙөДМјЛбёщАлЧУЕЁ¶ИПаөИЈ¬ёщҫЭЦКЧУКШәгФӯАнҝЙөГЈәc(H+)+c(H2CO3)=c(CO32Јӯ)+c(OHЈӯ)Ј¬№КDСЎПоХэИ·ЎЈ
ҝјөгЈәҝјІйЛ®ИЬТәЦРБЈЧУЕЁ¶ИҙуРЎ№ШПөЈ¬Йжј°СОАаЛ®ҪвіМ¶ИҙуРЎ№ШПөЎўСОИЬТәЦРЦКЧУКШәг№ШПөКҪЎўИЬТәөДpHЎўЛ®өДАлЧУ»эөИЎЈ
ұҫМвДС¶ИЈәТ»°г
| Ўҫҙу ЦР РЎЎҝЎҫҙтУЎЎҝ Ўҫ·ұМеЎҝ Ўҫ№ШұХЎҝ Ўҫ·ө»Ш¶ҘІҝЎҝ | |
| ПВТ»ЖӘЈәёЯҝј»ҜС§ұШҝјЦӘК¶өгЎ¶ҪрКфј°Жд»Ҝ.. | |
| Па№ШАёДҝ |