1ЎўСЎФсМв Т»¶ЁМхјюПВЈ¬»ҜС§·ҙУҰ2H2+O2ЁT2H2OөДДЬБҝұд»ҜИзНјЛщКҫЈ¬Фт·ҙУҰөДИИ»ҜС§·ҪіМКҪҝЙұнКҫОӘЈЁ ? Ј©
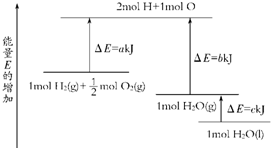
AЈ®H2ЈЁgЈ©+1/2O2ЈЁgЈ©ЁTH2OЈЁgЈ©ЎчH=ЈЁa+bЈ©kJ?mol-1
BЈ®2H2ЈЁgЈ©+O2ЈЁgЈ©ЁT2H2OЈЁgЈ©ЎчH=2ЈЁb-aЈ©kJ?mol-1
CЈ®H2ЈЁgЈ©+1/2O2ЈЁgЈ©ЁTH2OЈЁlЈ©ЎчH=ЈЁb+c-aЈ©kJ?mol-1
DЈ®2H2ЈЁgЈ©+O2ЈЁgЈ©ЁT2H2OЈЁlЈ©ЎчH=2ЈЁa-b-cЈ©kJ?mol-1
ІОҝјҙр°ёЈәD
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўМоҝХМв 0.3moLЖшМ¬ёЯДЬИјБПТТЕрНйЈЁB2H6Ј©ФЪСхЖшЦРИјЙХЈ¬ЙъіЙ№ММ¬B2O3әНТәМ¬Л®Ј¬·Еіц649.5KJИИБҝЈ¬ЗлРҙіцТТЕрНйИјЙХөДИИ»ҜС§·ҪіМКҪ______Ј®
ІОҝјҙр°ёЈә0.3molЖшМ¬ёЯДЬИјБПТТЕрНйФЪСхЖшЦРИјЙХЈ¬ЙъіЙ№ММ¬ИэСх»Ҝ¶юЕрәНТәМ¬Л®Ј¬·Еіц649.5KJөДИИБҝЈ¬Фт1molЖшМ¬ёЯДЬИјБПТТЕрНйФЪСхЖшЦРИјЙХЈ¬ЙъіЙ№ММ¬ИэСх»Ҝ¶юЕрәНТәМ¬Л®Ј¬·Еіц2165KJөДИИБҝЈ¬·ҙУҰөДИИ»ҜС§·ҪіМКҪОӘB2H6ЈЁgЈ©+3O2ЈЁgЈ©=B2O3ЈЁsЈ©+3H2OЈЁlЈ©ЎчH=-2165kJ/molЈ¬
№Кҙр°ёОӘЈәB2H6ЈЁgЈ©+3O2ЈЁgЈ©=B2O3ЈЁsЈ©+3H2OЈЁlЈ©ЎчH=-2165kJ/molЈ®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
3ЎўјтҙрМв ТСЦӘH2ЎўC2H4ЎўC2H6ёч1mol·ЦұрИјЙХЙъіЙТәМ¬Л®Ј¬·ЕіцИИБҝ·ЦұрОӘ285.8kJЎў1409.5kJЎў1558kJЈ®КФРҙіцC2H4ЙъіЙC2H6өДИИ»ҜС§·ҪіМКҪЈ®
ІОҝјҙр°ёЈәУЙМвТвҝЙРҙіцИИ»ҜС§·ҪіМКҪЈә
H2ЈЁgЈ©+12O2ЈЁgЈ©ЁTH2OЈЁlЈ©ЎчH=-285.8kJ?mol-1ўЩ
C2H4ЈЁgЈ©+3O2ЈЁgЈ©ЁT2CO2ЈЁgЈ©+2H2OЈЁlЈ©ЎчH=-1409.5kJ?mol-1ўЪ
C2H6ЈЁgЈ©+72O2ЈЁgЈ©ЁT2CO2ЈЁgЈ©+3H2OЈЁlЈ©ЎчH=-1558kJ?mol-ўЫ
ўЩ+ўЪ-ўЫҝЙөГC2H4ЈЁgЈ©+H2ЈЁgЈ©ЁTC2H6ЈЁgЈ©Ј¬
ЎчH=-285.8kJ?mol-1+ЈЁ-1409.5kJ?mol-1Ј©+1558kJ?mol-1=-137.3kJ?mol-1Ј®
ҙрЈәC2H4ЙъіЙC2H6өДИИ»ҜС§·ҪіМКҪОӘЈәC2H4ЈЁgЈ©+H2ЈЁgЈ©ЁTC2H6ЈЁgЈ©ЎчH=-137.3kJ?mol-1Ј®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
4ЎўСЎФсМв SF6КЗТ»ЦЦУЕБјөДЖшМеҫшФөІДБПЈ¬·ЦЧУҪб№№ЦРЦ»ҙжФЪSЎӘFјьЎЈ·ўЙъ·ҙУҰөДИИ»ҜС§·ҪіМКҪОӘЈәS(s)Ј«3F2(g) = SF6(g) ҰӨH="
ІОҝјҙр°ёЈә
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
5ЎўМоҝХМв 4ҝЛБт·ЫФЪСхЖшЦРНкИ«ИјЙХКұ·Еіц37З§Ҫ№ИИБҝЈ¬ёГ·ҙУҰөДИИ»ҜС§·ҪіМКҪКЗЈә_____________ ЎЈ
ІОҝјҙр°ёЈәЎ°ВФЎұ
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г