1ЎўјтҙрМв ТСЦӘH2ЎўC2H4ЎўC2H6ёч1mol·ЦұрИјЙХЙъіЙТәМ¬Л®Ј¬·ЕіцИИБҝ·ЦұрОӘ285.8kJЎў1409.5kJЎў1558kJЈ®КФРҙіцC2H4ЙъіЙC2H6өДИИ»ҜС§·ҪіМКҪЈ®
ІОҝјҙр°ёЈәУЙМвТвҝЙРҙіцИИ»ҜС§·ҪіМКҪЈә
H2ЈЁgЈ©+12O2ЈЁgЈ©ЁTH2OЈЁlЈ©ЎчH=-285.8kJ?mol-1ўЩ
C2H4ЈЁgЈ©+3O2ЈЁgЈ©ЁT2CO2ЈЁgЈ©+2H2OЈЁlЈ©ЎчH=-1409.5kJ?mol-1ўЪ
C2H6ЈЁgЈ©+72O2ЈЁgЈ©ЁT2CO2ЈЁgЈ©+3H2OЈЁlЈ©ЎчH=-1558kJ?mol-ўЫ
ўЩ+ўЪ-ўЫҝЙөГC2H4ЈЁgЈ©+H2ЈЁgЈ©ЁTC2H6ЈЁgЈ©Ј¬
ЎчH=-285.8kJ?mol-1+ЈЁ-1409.5kJ?mol-1Ј©+1558kJ?mol-1=-137.3kJ?mol-1Ј®
ҙрЈәC2H4ЙъіЙC2H6өДИИ»ҜС§·ҪіМКҪОӘЈәC2H4ЈЁgЈ©+H2ЈЁgЈ©ЁTC2H6ЈЁgЈ©ЎчH=-137.3kJ?mol-1Ј®
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
2ЎўСЎФсМв ФЪ298KЎў101kPaКұЈ¬ТСЦӘЈә
2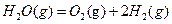 ЎчH1
ЎчH1
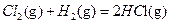 ЎчH2
ЎчH2
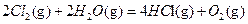 ЎчH3
ЎчH3
Фт
ІОҝјҙр°ёЈә
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
3ЎўСЎФсМв ПВБРЛө·Ё»тұнКҫ·ЁХэИ·өДКЗ
[? ]
AЈ®өИБҝөДБтХфЖшәНБт№ММе·ЦұрНкИ«ИјЙХЈ¬әуХЯ·ЕіцИИБҝ¶а
BЈ®УЙC(КҜД«)ЎъC(ҪрёХКҜ) ҰӨH = +1Ј®9 kJЎӨmol-1ҝЙЦӘЈ¬КҜД«ұИҪрёХКҜОИ¶Ё
CЈ®ФЪПЎИЬТәЦРЈәH++OH-==H2O ҰӨH = Јӯ57Ј®3 kJЎӨ mol-1Ј¬ИфҪ«ә¬1mol CH3COOHөДҙЧЛбИЬТәУлә¬1mol
NaOHөДИЬТә»мәПЈ¬·ЕіцөДИИБҝҙуУЪ57Ј®3kJ
DЈ®ФЪ101 kPaКұЈ¬2g H2НкИ«ИјЙХЙъіЙТәМ¬Л®Ј¬·Еіц285Ј®8 kJИИБҝЈ¬ЗвЖшИјЙХөДИИ»ҜС§·ҪіМКҪұнКҫОӘ
2H2ЈЁgЈ©+ O2ЈЁgЈ©==2H2OЈЁlЈ© ҰӨH = +285Ј®8 kJЎӨ mol-1
ІОҝјҙр°ёЈәB
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәТ»°г
4ЎўМоҝХМв ТСЦӘЈәўЩ N2 (g) + 3H2 (g)  2NH3 (g) ҰӨHЈҪЈӯ92.4 kJ/mol
2NH3 (g) ҰӨHЈҪЈӯ92.4 kJ/mol
ўЪ 2H2 (g)+ SO2 (g)  S (g) + 2H2O (g) ҰӨH ЈҪ+90.4 kJ/mol
S (g) + 2H2O (g) ҰӨH ЈҪ+90.4 kJ/mol
ўЫ 2H2 (g) + CO (g)  CH3OH (g) ҰӨHЈҪЈӯ90.8 kJ/mol
CH3OH (g) ҰӨHЈҪЈӯ90.8 kJ/mol
Зл»ШҙрЈә
ЈЁ1Ј©ЙПКцұд»Ҝ№эіМЦР·ЕіцИИБҝөДКЗ ЈЁМоРтәЕЈ¬ПВН¬Ј©Ј¬ОьКХИИБҝөДКЗ ЎЈ
ЈЁ2Ј©4 g H2 (g) НкИ«·ҙУҰЙъіЙјЧҙјЖшМеКұЈ¬ҰӨH = kJ/molЎЈ
ЈЁ3Ј©ЙъіЙ1 mol NH3 (g) өД·ЕіцөДИИБҝОӘ kJЎЈ
ІОҝјҙр°ёЈәЈЁ1Ј©ўЩўЫЈ¬ўЪЎЈЈЁ2Ј©Ј®Јӯ90.8Ј» ЈЁ3Ј©46.2
ұҫМвҪвОцЈәЈЁ1Ј©·ЕіцИИБҝөД·ҙУҰИИҰӨHЈј0Ј¬ОьКХИИБҝөД·ҙУҰИИОӘҰӨHЈҫ0Ј¬ЛщТФЙПКцұд»Ҝ№эіМЦР·ЕіцИИБҝөДКЗўЩўЫЈ¬ОьКХИИБҝөДКЗўЪЎЈ
ЈЁ2Ј©УЙИИ»ҜС§·ҙУҰ·ҪіМКҪ2H2 (g) + CO (g)  CH3OH (g) ҰӨHЈҪЈӯ90.8 kJ/molҝЙөГ4 g H2 (g) НкИ«·ҙУҰЙъіЙјЧҙјЖшМеКұЈ¬ҰӨH =Јӯ90.8 kJ/molЎЈ
CH3OH (g) ҰӨHЈҪЈӯ90.8 kJ/molҝЙөГ4 g H2 (g) НкИ«·ҙУҰЙъіЙјЧҙјЖшМеКұЈ¬ҰӨH =Јӯ90.8 kJ/molЎЈ
ЈЁ3Ј©УЙИИ»ҜС§·ҙУҰ·ҪіМКҪN2 (g) + 3H2 (g)  2NH3 (g) ҰӨHЈҪЈӯ92.4 kJ/molҝЙөГЙъіЙ1 mol NH3 (g) өД·ЕіцөДИИБҝОӘ46.2 kJЎЈ
2NH3 (g) ҰӨHЈҪЈӯ92.4 kJ/molҝЙөГЙъіЙ1 mol NH3 (g) өД·ЕіцөДИИБҝОӘ46.2 kJЎЈ
ұҫМвДС¶ИЈәТ»°г
5ЎўМоҝХМв өӘЖшј°ә¬өӘөД»ҜәПОпФЪ№ъГсҫӯјГЦРХјУРЦШТӘөШО»ЎЈәПіЙ°ұ№ӨТөЦРЈ¬әПіЙЛюЦРГҝІъЙъ2 mol NH3Ј¬·Еіц92Ј®4 kJИИБҝЎЈ
ЈЁ1Ј©ИфЖрКјКұПтИЭЖчДЪ·ЕИл2 mol N2әН6 mol H2Ј¬ҙпЖҪәвәу·ЕіцөДИИБҝОӘQЈ¬ФтQ 184Ј®8kJЈЁМоЎ°>ЎұЎўЎ°<Ўұ»тЎ°=ЎұЈ©ЎЈТ»¶ЁМхјюПВЈ¬ФЪГЬұХәгИЭөДИЭЖчЦРЈ¬ДЬұнКҫ·ҙУҰҙпөҪ»ҜС§ЖҪәвЧҙМ¬өДКЗ ЎЈ
aЈ®3vДж(N2)=vХэ(H2Ј© bЈ®2vХэ(H2)= vХэ(NH3Ј©
cЈ®»мәПЖшМеГЬ¶ИұЈіЦІ»ұд dЈ®c(N2)Јәc(H2)Јәc(NH3)=1Јә3Јә2
№ӨТөЙъІъДтЛШөДФӯАнКЗТФNH3әНCO2ОӘФӯБПәПіЙДтЛШ[CO(NH2)2]Ј¬·ҙУҰөД»ҜС§·ҪіМКҪОӘ
2NH3 (g)+ CO2 (gЈ© CO(NH2)2 (lЈ©+ H2O (l)ЎЈ
CO(NH2)2 (lЈ©+ H2O (l)ЎЈ
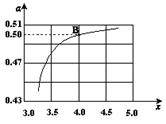
ЈЁ2Ј©ФЪТ»¶ЁОВ¶ИәНС№ЗҝПВЈ¬ИфФӯБПЖшЦРөДNH3әНCO2өДОпЦКөДБҝЦ®ұИЈЁ°ұМјұИЈ©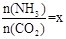 Ј¬УТНјКЗ°ұМјұИЈЁxЈ©УлCO2ЖҪәвЧӘ»ҜВКЈЁҰБЈ©өД№ШПөЎЈҰБЛжЧЕxФцҙу¶шФцҙуөДФӯТтКЗ ЎЈ
Ј¬УТНјКЗ°ұМјұИЈЁxЈ©УлCO2ЖҪәвЧӘ»ҜВКЈЁҰБЈ©өД№ШПөЎЈҰБЛжЧЕxФцҙу¶шФцҙуөДФӯТтКЗ ЎЈ
ЈЁ3Ј©НјЦРөДBөгҙҰЈ¬NH3өДЖҪәвЧӘ»ҜВКОӘ ЎЈ
ЈЁ4Ј©ТСЦӘЈә 3Cl2+2NH3ЎъN2+6HCl
ІОҝјҙр°ёЈә
ұҫМвҪвОцЈә
ұҫМвДС¶ИЈәА§ДС