1ЎўСЎФсМв ПВБРУР№Ш»ҜС§УГУпұнКҫХэИ·өДКЗЈЁЎЎЎЎЈ©
AЈ®HClO·ЦЧУөДҪб№№КҪЈәH-O-Cl
BЈ®ТТИІ·ЦЧУөДөзЧУКҪЈә

CЈ®CЎўҰБ-°ұ»щұыЛб·ЦЧУөДҪб№№јтКҪЈәH2NCH2CH2COOH
DЈ®NaHCO3өДөзАл·ҪіМКҪЈәNaHCO3ЁTNa++H++CO
2ЎўМоҝХМв ЈЁ14Ј©AЎўBЎўCЎўDЎўEЎўFКЗіЈјыөҘЦКЈ¬ЖдЦРAКЗУГБҝЧоҙуЈ¬УҰУГЧо№г·әөДҪрКфЈ»ФӘЛШDКЗөШҝЗЦРә¬БҝЧо¶аөДҪрКфФӘЛШЈ»DФӘЛШәНEФӘЛШФЪЦЬЖЪұнЦРПаБЪЎЈGЎўHЎўIЎўJЎўKЎўLКЗіЈјы»ҜәПОпЈ¬ЖдЦРGФЪіЈОВПВКЗОЮЙ«ТәМеЈ¬HКЗәЪЙ«№ММеЎЈТФЙПОпЦКПа»ҘјдөДЧӘ»Ҝ№ШПөИзНјЛщКҫЈә
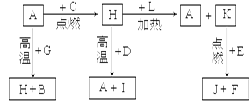
Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ФӘЛШAФЪФӘЛШЦЬЖЪұнЦРөДО»ЦГОӘ??ЦЬЖЪ??ЧеЎЈ
ЈЁ2Ј©РҙіцKөДөзЧУКҪ??ЎЈ
ЈЁ3Ј©РҙіцHәНD·ҙУҰөД»ҜС§·ҪіМКҪ??ЎЈ
ЈЁ4Ј©DДЬУлЗвСх»ҜДЖИЬТә·ҙУҰЈ¬ЗлРҙіцёГ·ҙУҰөДАлЧУ·ҪіМКҪ??ЎЈ
ЈЁ5Ј©РҙіцAУлG·ҙУҰөД»ҜС§·ҪіМКҪ??ЎЈ
ЈЁ6Ј©KУлE·ҙУҰКұІъЙъөДПЦПуУР?Ј»ёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘ? ___________________ЎЈ
ЈЁ7Ј©ТСЦӘFөДИјЙХИИОӘakJ/mol,LөДИјЙХИИОӘbkJ/mol,КФРҙіцFЙъіЙLөДИИ»ҜС§·ҪіМКҪ_________________
3ЎўСЎФсМв CuУлZnУГөјПЯБ¬ҪУәуҪюИлПЎБтЛбЧйіЙФӯөзіШЧ°ЦГЎЈөұөјПЯЦРУР0.5molөзЧУНЁ№эКұЈ¬АнВЫЙПБҪј«өДұд»ҜХэИ·өДКЗ
AЈ®РҝОӘёәј«Ј¬РҝЖ¬ИЬҪв32.5g
BЈ®НӯОӘХэј«Ј¬НӯЖ¬ЙПІъЙъ0.25molөДH2
CЈ®өзЧУУЙНӯј«ҫӯөјПЯБчПтРҝј«
DЈ®ИЬТәЦРSO42-¶ЁПтТЖПтНӯј«