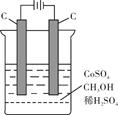
1ЎўјтҙрМв ТСЦӘПВБРИИ»ҜС§·ҪіМКҪЈә
ЈЁ1Ј©CЈЁsЈ¬КҜД«Ј©+O2ЈЁgЈ©ЁTCO2ЈЁgЈ©ЎчH=-393.5kJ?mol-1
ЈЁ2Ј©2H2ЈЁgЈ©+O2ЈЁgЈ©ЁT2H2OЈЁlЈ©ЎчH=-571.6kJ?mol-1
ЈЁ3Ј©2C2H2ЈЁgЈ©+5O2ЈЁgЈ©ЁT4CO2ЈЁgЈ©+2H2OЈЁlЈ©ЎчH=-2599kJ?mol-1
ЗлРҙіцCЈЁsЈ¬КҜД«Ј©әНH2ЈЁgЈ©ЙъіЙ1mol C2H2ЈЁgЈ©өДИИ»ҜС§·ҪіМКҪ______Ј®
2ЎўСЎФсМв ТСЦӘ101KPaКұРБНйөДұкЧјИјЙХИИОӘЎӘ5518kJЎӨmol-1Ј¬ә¬20gNaOHөДПЎИЬТәУлПЎСОЛбід·Ц·ҙУҰ·ЕіцИИБҝ28.7kJЈ¬ФтПВБРИИ»ҜС§·ҪіМКҪКйРҙХэИ·өДКЗ
ўЩC8H18ЈЁlЈ©+ 25/2O2ЈЁgЈ©ЈҪ 8CO2ЈЁgЈ©+ 9H2OЈЁgЈ©Ј» ЎчH ЈҪ +5518 kJЎӨmol-1
ўЪC8H18ЈЁlЈ©+ 25/2O2ЈЁgЈ©ЈҪ 8CO2ЈЁgЈ©+ 9H2OЈЁlЈ©Ј» ЎчH ЈҪ Јӯ5518 kJЎӨmol-1
ўЫH+ЈЁaqЈ©+ OH-ЈЁaqЈ©ЈҪ H2OЈЁlЈ©Ј» ЎчH ЈҪ Јӯ57Ј®4 kJЎӨmol-1
ўЬHCl (aq) + NaOH(aq) ЈҪNaCl(aq) + H2O(l)Ј» ЎчH ЈҪ ЎӘ28.7 kJЎӨmol-1
AЈ®ўЩўЫ
BЈ®ўЪўЫ
CЈ®ўЪўЬ
DЈ®ўЪ